Epidemiological Profile of Hospitalized Patients with Cystic Fibrosis in Brazil Due to Severe Acute Respiratory Infection during the COVID-19 Pandemic and a Systematic Review of Worldwide COVID-19 in Those with Cystic Fibrosis
- PMID: 37444770
- PMCID: PMC10341451
- DOI: 10.3390/healthcare11131936
Epidemiological Profile of Hospitalized Patients with Cystic Fibrosis in Brazil Due to Severe Acute Respiratory Infection during the COVID-19 Pandemic and a Systematic Review of Worldwide COVID-19 in Those with Cystic Fibrosis
Abstract
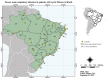
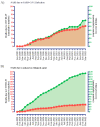
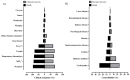
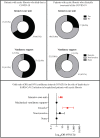
Since the onset of the coronavirus disease, COVID-19 pandemic, concern arose for those who might be at higher risk of a worse COVID-19 prognosis, such as those with cystic fibrosis (CF). In this context, we evaluated the features of hospitalized patients with CF due to severe acute respiratory infection (SARI) in Brazil and we also performed a systematic review including all the studies published from the beginning of the first case of COVID-19 (17 November 2019) to the date of this search (23 May 2022) which included, concomitantly, patients with CF and COVID-19 in the worldwide population. In our Brazilian data, we evaluated the period from December 2019 to March 2022, and we included 33 demographical and clinical patients' features. We classified the patients into groups: (G1) SARI due to another viral infection than severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (23; 5.4%), (G2) SARI due to an unknown etiological agent (286; 67.1%), and (G3) SARI due to SARS-CoV-2 infection (117; 27.5%). The individuals in G3 tended to be older, especially over 50 years old, and presented a higher prevalence of dyspnea, peripheral capillary oxygen saturation (SpO2) <95%, and cardiopathy. The highest prevalence for intensive care unit (ICU) treatment (52; 44.4%) and invasive mechanical ventilation (29; 24.8%) was for patients in G3. Almost half of the patients in G3 died (51; 43.6%); in contrast, none in G1 died. However, we observed 43 (15.0%) deaths in G2. In addition, 12 (4.2%) and one (0.9%) death not associated with SARI occurred, respectively, in the G2 and G3. The patients who died due to SARS-CoV-2 infection had a higher frequency of SpO2 <95% (46; 90.2%), ICU treatment (34; 66.7%), and invasive mechanical ventilation (27; 52.9%) when compared to those who recovered. The systematic review comprised a total of 31 papers published as observational studies. These studies comprised 661,386 patients in total, including children, adults, and elderly age groups. However, only 19,150 (2.9%) patients were diagnosed with CF and, from these patients, 2523 (0.4%) were diagnosed with both CF and COVID-19. It was observed that the most common outcome was the need for hospitalization (n = 322 patients with CF), and the need for oxygen support (n = 139 patients with CF). One hundred patients with CF needed intensive care units, fifty patients needed non-invasive mechanical ventilation support, and only three patients were described as receiving invasive mechanical ventilation support. Deaths were described in 38 patients with CF. Importantly, lung-transplanted patients with CF represented an increased risk of death in one publication; in accordance, another study described that lung transplantation and moderate to severe lung disease were independent risk factors for severe outcomes after SARS-CoV-2 infection. In contrast with the literature, in conclusion, Brazilian patients in G3 presented a severe phenotype, even though most of the other studies did not observe worse outcomes in patients with CF and COVID-19.
Keywords: Brazil; CFTR; SARS-CoV-2; epidemiology; mucoviscidosis; p.Phe508del; severity; systematic review; worldwide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Epidemiologic Profile of Severe Acute Respiratory Infection in Brazil During the COVID-19 Pandemic: An Epidemiological Study.Front Microbiol. 2022 Jul 1;13:911036. doi: 10.3389/fmicb.2022.911036. eCollection 2022. Front Microbiol. 2022. PMID: 35854935 Free PMC article.
-
Severe Acute Respiratory Syndrome by SARS-CoV-2 Infection or Other Etiologic Agents Among Brazilian Indigenous Population: An Observational Study from the First Year of Coronavirus Disease (COVID)-19 Pandemic.Lancet Reg Health Am. 2022 Apr;8:100177. doi: 10.1016/j.lana.2021.100177. Epub 2022 Jan 7. Lancet Reg Health Am. 2022. PMID: 35018359 Free PMC article.
-
Clinical characteristics and comorbidities of COVID-19 in unvaccinated patients with Down syndrome: first year report in Brazil.Hum Genet. 2022 Dec;141(12):1887-1904. doi: 10.1007/s00439-022-02468-3. Epub 2022 Jun 28. Hum Genet. 2022. PMID: 35763088 Free PMC article.
-
Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic.BMC Pulm Med. 2021 May 20;21(1):173. doi: 10.1186/s12890-021-01528-0. BMC Pulm Med. 2021. PMID: 34016096 Free PMC article.
-
Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit.JAMA Netw Open. 2020 Jun 1;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. JAMA Netw Open. 2020. PMID: 32543702 Free PMC article. Review.
Cited by
-
Demographic and Clinical Profile of Patients with Osteogenesis Imperfecta Hospitalized Due to Coronavirus Disease (COVID)-19: A Case Series of 13 Patients from Brazil.Healthcare (Basel). 2025 Jul 23;13(15):1779. doi: 10.3390/healthcare13151779. Healthcare (Basel). 2025. PMID: 40805812 Free PMC article.
-
Occurrence of COVID-19 in cystic fibrosis patients: a review.Front Microbiol. 2024 Apr 17;15:1356926. doi: 10.3389/fmicb.2024.1356926. eCollection 2024. Front Microbiol. 2024. PMID: 38694803 Free PMC article. Review.
-
Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis.Int J Mol Sci. 2024 Mar 27;25(7):3713. doi: 10.3390/ijms25073713. Int J Mol Sci. 2024. PMID: 38612524 Free PMC article. Review.
-
Were deaths recorded in Brazil due to cystic fibrosis or pulmonary fibrosis? A data-based analysis.Front Med (Lausanne). 2024 Aug 21;11:1459785. doi: 10.3389/fmed.2024.1459785. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39253539 Free PMC article. No abstract available.
References
-
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

