Impact of a Digital Tool on Pharmacy Students' Ability to Perform Medication Reviews: A Randomized Controlled Trial
- PMID: 37444802
- PMCID: PMC10341599
- DOI: 10.3390/healthcare11131968
Impact of a Digital Tool on Pharmacy Students' Ability to Perform Medication Reviews: A Randomized Controlled Trial
Abstract
Digital Medication Review Tools (DMRTs) are increasingly important in pharmacy practice. To ensure that young pharmacists are sufficiently competent to perform medication reviews after graduation, the introduction of DMRTs teaching in academic education is necessary. The aim of our study was to demonstrate the effect of DMRTs use on pharmacy students' performance when conducting a medication review (MR) in a randomized controlled pre-post design. Forty-one pharmacy students were asked to complete a MR within 60 min, followed by a 10-min consultation with (intervention group) and without a DMRT (control group). The MR performance was subdivided into four categories: communication skills, subjective and objective patient data, assessment, and plan. Performance was assessed using objective structured clinical examinations (OSCEs) and analytical checklists. With the use of DMRTs, the overall performance was improved by 17.0% compared to the control group (p < 0.01). Improvement through DMRTs was seen in the subcategories "Assessment" and "Plan". Furthermore, pharmacy students liked using DMRTs and felt more confident overall. Our study results demonstrate that DMRTs improve the performance of MRs, hence DMRTs should become an integral part of pharmacy curriculum. Consequently, digitally enabled pharmacists using DMRTs will be better prepared for their professional careers in pharmacy practice.
Keywords: digital health; digital tool; eHealth; medication review; medication safety; pharmaceutical services; pharmacy education.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures



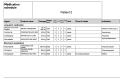



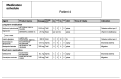

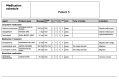



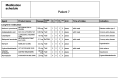
























References
-
- Seidling H.M., Send A.F.J., Bittmann J., Renner K., Dewald B., Lange D., Bruckner T., Haefeli W.E. Medication review in German community pharmacies—Post-hoc analysis of documented drug-related problems and subsequent interventions in the ATHINA-project. Res. Soc. Adm. Pharm. 2017;13:1127–1134. doi: 10.1016/j.sapharm.2016.10.016. - DOI - PubMed
-
- Federal Union of German Associations of Pharmacists Pharmazeutische Dienstleistungen. 2022. [(accessed on 20 December 2022)]. Available online: https://www.abda.de/pharmazeutische-dienstleistungen/
Grants and funding
LinkOut - more resources
Full Text Sources

