Thermal Decomposition Path-Studied by the Simultaneous Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy and Quadrupole Mass Spectrometry-Of Imidazoline/Dimethyl Succinate Hybrids and Their Biological Characterization
- PMID: 37444951
- PMCID: PMC10342989
- DOI: 10.3390/ma16134638
Thermal Decomposition Path-Studied by the Simultaneous Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy and Quadrupole Mass Spectrometry-Of Imidazoline/Dimethyl Succinate Hybrids and Their Biological Characterization
Abstract
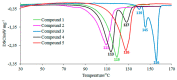
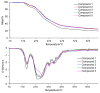
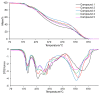
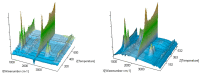
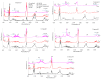
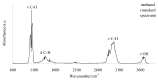
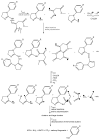
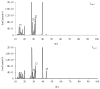
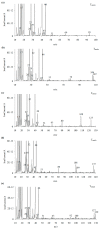
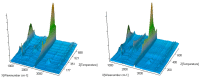
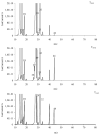
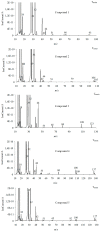
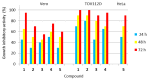
The thermal decomposition path of synthetically and pharmacologically useful hybrid materials was analyzed in inert and oxidizing conditions for the first time and presented in this article. All the imidazoline/dimethyl succinate hybrids (1-5) were studied using the simultaneous thermogravimetry (TG) coupled with Fourier transform infrared spectroscopy (FTIR) and quadrupole mass spectrometry (QMS). It was found that the tested compounds were thermally stable up to 200-208 °C (inert conditions) and up to 191-197 °C (oxidizing conditions). In both furnace atmospheres, their decomposition paths were multi-step processes. At least two major stages (inert conditions) and three major stages (oxidizing conditions) of their decomposition were observed. The first decomposition stage occurred between T5% and 230-237 °C. It was connected with the breaking of one ester bond. This led to the emission of one methanol molecule and the formation of radicals capable of further radical reactions in both used atmospheres. At the second decomposition stage (Tmax2) between 230-237 °C and 370 °C (inert conditions), or at about 360 °C (oxidizing conditions), the cleavage of the second ester bond and N-N and C-C bonds led to the emission of CH3OH, HCN, N2, and CO2 and other radical fragments that reacted with each other to form clusters and large clusters. Heating the tested compounds to a temperature of about 490 °C resulted in the emission of NH3, HCN, HNCO, aromatic amines, carbonyl fragments, and the residue (Tmax2a) in both atmospheres. In oxidizing conditions, the oxidation of the formed residues (Tmax3) was related to the production of CO2, CO, and H2O. These studies confirmed the same radical decomposition mechanism of the tested compounds both in inert and oxidizing conditions. The antitumor activities and toxicities to normal cells of the imidazoline/dimethyl succinate hybrids were also evaluated. As a result, the two hybrid materials (3 and 5) proved to be the most selective in biological studies, and therefore, they should be utilized in further, more extended in vivo investigations.
Keywords: antihemolytic activity; antitumor activity; decomposition course; imidazoline/dimethyl succinate hybrids; thermal behavior; toxicity to normal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
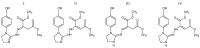

Similar articles
-
Experimental Studies on the Thermal Properties and Decomposition Course of a Novel Class of Heterocyclic Anticancer Drug Candidates.Int J Mol Sci. 2023 Mar 24;24(7):6190. doi: 10.3390/ijms24076190. Int J Mol Sci. 2023. PMID: 37047158 Free PMC article.
-
In Vitro, In Vivo, Ex Vivo Characterisation of Dihydroimidazotriazinones and Their Thermal Decomposition Course Studied by Coupled and Simultaneous Thermal Analysis Methods.Int J Mol Sci. 2025 Jan 10;26(2):541. doi: 10.3390/ijms26020541. Int J Mol Sci. 2025. PMID: 39859257 Free PMC article.
-
Studies on the Thermal Decomposition Course of Nitrogen-Rich Heterocyclic Esters as Potential Drug Candidates and Evaluation of Their Thermal Stability and Properties.Int J Mol Sci. 2024 Apr 27;25(9):4768. doi: 10.3390/ijms25094768. Int J Mol Sci. 2024. PMID: 38731989 Free PMC article.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
Coupled and Simultaneous Thermal Analysis Techniques in the Study of Pharmaceuticals.Pharmaceutics. 2023 May 25;15(6):1596. doi: 10.3390/pharmaceutics15061596. Pharmaceutics. 2023. PMID: 37376045 Free PMC article. Review.
Cited by
-
First voltammetric analysis of two possible anticancer drug candidates using an unmodified glassy carbon electrode.Sci Rep. 2024 Jul 27;14(1):17306. doi: 10.1038/s41598-024-68309-7. Sci Rep. 2024. PMID: 39068200 Free PMC article.
References
-
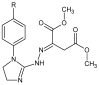
- Sztanke K., Tkaczyński T. Preparation of New Dimethyl Esters of 2-[(1-aryl-Δ2-imidazolin-2-ylhydrazono]succinic Acids. 188616. Polish Patent. 2005 March 31;
-
- Caires C.F.J., Lima L.S., Carvalho C.T., Ionashiro M. Thermal behaviour of succinic acid, sodium succinate and its compounds with some bivalent transitions metal ions in dynamic N2 and CO2 atmospheres. Eclética Química São Paulo. 2010;35:73–80. doi: 10.1590/S0100-46702010000400009. - DOI
-
- Caires C.F.J., Lima L.S., Carvalho C.T., Ionashiro M. Thermal behaviour of succinic acid, sodium succinate and its compounds with some bivalent transition metal ions. Thermochim. Acta. 2010;500:6–12. doi: 10.1016/j.tca.2009.11.015. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

