Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements; Recent Trends in Preservation and Legislation
- PMID: 37445107
- PMCID: PMC10343617
- DOI: 10.3390/ma16134793
Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements; Recent Trends in Preservation and Legislation
Abstract
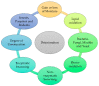
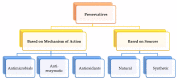
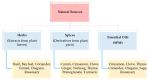
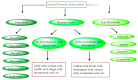
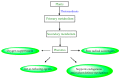
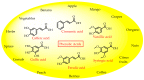
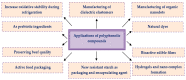
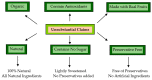
The global market of food, cosmetics, and pharmaceutical products requires continuous tracking of harmful ingredients and microbial contamination for the sake of the safety of both products and consumers as these products greatly dominate the consumer's health, directly or indirectly. The existence, survival, and growth of microorganisms in the product may lead to physicochemical degradation or spoilage and may infect the consumer at another end. It has become a challenge for industries to produce a product that is safe, self-stable, and has high nutritional value, as many factors such as physical, chemical, enzymatic, or microbial activities are responsible for causing spoilage to the product within the due course of time. Thus, preservatives are added to retain the virtue of the product to ensure its safety for the consumer. Nowadays, the use of synthetic/artificial preservatives has become common and has not been widely accepted by consumers as they are aware of the fact that exposure to preservatives can lead to adverse effects on health, which is a major area of concern for researchers. Naturally occurring phenolic compounds appear to be extensively used as bio-preservatives to prolong the shelf life of the finished product. Based on the convincing shreds of evidence reported in the literature, it is suggested that phenolic compounds and their derivatives have massive potential to be investigated for the development of new moieties and are proven to be promising drug molecules. The objective of this article is to provide an overview of the significant role of phenolic compounds and their derivatives in the preservation of perishable products from microbial attack due to their exclusive antioxidant and free radical scavenging properties and the problems associated with the use of synthetic preservatives in pharmaceutical products. This article also analyzes the recent trends in preservation along with technical norms that regulate the food, cosmetic, and pharmaceutical products in the developing countries.
Keywords: antioxidant; microbial activities; natural phenolic compounds; preservatives; radical scavenging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Carstensen J.T., Rhodes C. Drug stability, revised, and expanded: Principles and practices. Inf. Health Care. 2000;213:223.
-
- Khatkar A., Nanda A., Narasimhan B. Preservatives-associated problems and possible alternatives. Curr. Trends Biotechnol. 2012;2012:100–120.
-
- Anand S.P., Sati N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013;4:2496.
-
- Yoshioka S., Stella V.J. Stability of Drugs and Dosage Form. Adv. Drug Deliv. Rev. 2002;54:273–288.
-
- Aulton M.E., Taylor K.M. The Design and Manufacture of Medicines. Elsevier Health Sciences: Churchill Livingstone; Edinburgh, Scotland: 2013.
Publication types
LinkOut - more resources
Full Text Sources

