High-Efficiency Removal of Lead and Nickel Using Four Inert Dry Biomasses: Insights into the Adsorption Mechanisms
- PMID: 37445198
- PMCID: PMC10343542
- DOI: 10.3390/ma16134884
High-Efficiency Removal of Lead and Nickel Using Four Inert Dry Biomasses: Insights into the Adsorption Mechanisms
Abstract
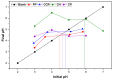
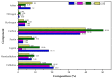
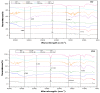
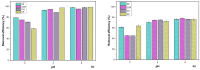
In this study, inert dry bioadsorbents prepared from corn cob residues (CCR), cocoa husk (CH), plantain peels (PP), and cassava peels (CP) were used as adsorbents of heavy metal ions (Pb2+ and Ni2+) in single-batch adsorption experiments from synthetic aqueous solutions. The physicochemical properties of the bioadsorbents and the adsorption mechanisms were evaluated using different experimental techniques. The results showed that electrostatic attraction, cation exchange, and surface complexation were the main mechanisms involved in the adsorption of metals onto the evaluated bioadsorbents. The percentage removal of Pb2+ and Ni2+ increased with higher adsorbent dosage, with Pb2+ exhibiting greater biosorption capacity than Ni2+. The bioadsorbents showed promising potential for adsorbing Pb2+ with monolayer adsorption capacities of 699.267, 568.794, 101.535, and 116.820 mg/g when using PP, CCR, CH, and CP, respectively. For Ni2+, Langmuir's parameter had values of 10.402, 26.984, 18.883, and 21.615, respectively, for PP, CCR, CH, and CP. Kinetics data fitted by the pseudo-second-order model revealed that the adsorption rate follows this order: CH > CP > CCR > PP for Pb2+, and CH > CCR > PP > CP for Ni2+. The adsorption mechanism was found to be controlled by ion exchange and precipitation. These findings suggest that the dry raw biomasses of corn cob residues, cocoa husk, cassava, and plantain peels can effectively remove lead and nickel, but further research is needed to explore their application in industrial-scale and continuous systems.
Keywords: adsorption mechanism; bioadsorption; heavy metal ions; isotherms; kinetics; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Optimization and mechanisms of methylene blue removal by foxtail millet shell from aqueous water and reuse in biosorption of Pb(II), Cd(II), Cu(II), and Zn(II) for secondary times.Int J Phytoremediation. 2022;24(4):350-363. doi: 10.1080/15226514.2021.1944978. Epub 2021 Aug 19. Int J Phytoremediation. 2022. PMID: 34410866
-
Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification.J Hazard Mater. 2020 Apr 5;387:121718. doi: 10.1016/j.jhazmat.2019.121718. Epub 2019 Nov 19. J Hazard Mater. 2020. PMID: 31771887
-
Facile preparation of amine -functionalized corn husk derived activated carbon for effective removal of selected heavy metals from battery recycling wastewater.Heliyon. 2022 May 23;8(5):e09516. doi: 10.1016/j.heliyon.2022.e09516. eCollection 2022 May. Heliyon. 2022. PMID: 35663746 Free PMC article.
-
Sustainable approaches for nickel removal from wastewater using bacterial biomass and nanocomposite adsorbents: A review.Chemosphere. 2022 Mar;291(Pt 1):132862. doi: 10.1016/j.chemosphere.2021.132862. Epub 2021 Nov 10. Chemosphere. 2022. PMID: 34774612 Review.
-
A Review on Advances in the Use of Raw and Modified Agricultural Lignocellulosic Residues in Mono- and Multicomponent Continuous Adsorption of Inorganic Pollutants for Upscaling Technologies.Polymers (Basel). 2025 Mar 31;17(7):953. doi: 10.3390/polym17070953. Polymers (Basel). 2025. PMID: 40219342 Free PMC article. Review.
Cited by
-
Evaluating and Selecting Kinetic and Isotherm Models for Copper and Nickel Removal Using Cow Bone Char as an Adsorbent via Excel Solver Functions.Int J Mol Sci. 2025 May 1;26(9):4316. doi: 10.3390/ijms26094316. Int J Mol Sci. 2025. PMID: 40362556 Free PMC article.
References
-
- Freitas L.C., Barbosa J.R., da Costa A.L.C., Bezerra F.W.F., Pinto R.H.H., de Junior R.N.C. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021;169:105466. doi: 10.1016/j.resconrec.2021.105466. - DOI
-
- Vega Cuellar M.Á., Calderon Dominguez G., Perea Flores MD J., Pena Barrientos A., Salgado Cruz MD L.P., Garcia Hernandez A.B., Davila Ortiz G. Use of microorganisms and agro-industrial wastes in the biosorption of chromium (VI): A review. Waste Biomass Valorization. 2022;13:4115–4136. doi: 10.1007/s12649-022-01755-4. - DOI
-
- de Oliveira A.V.B., Rizzato T.M., Barros B.C.B., Favaro S.L., Caetano W., Hioka N., Batistela V.R. Physicochemical modifications of sugarcane and cassava agro-industrial wastes for applications as biosorbents. Bioresour. Technol. Rep. 2019;7:100294. doi: 10.1016/j.biteb.2019.100294. - DOI
-
- Kumar V., Parihar R.D., Sharma A., Bakshi P., Sidhu G.P.S., Bali A.S., Karaouzas I., Bhardwaj R., Thukral A.K., Gyasi-Agyei Y., et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere. 2019;236:124364. doi: 10.1016/j.chemosphere.2019.124364. - DOI - PubMed
-
- Li Y., Zhou Q., Ren B., Luo J., Yuan J., Ding X., Bian H., Yao X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. Rev. Environ. Contam. Toxicol. 2020;251:1–24. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

