Transoral Laser Microsurgery versus Robot-Assisted Surgery for Squamous Cell Carcinoma of the Tongue Base (Oncological and Functional Results)-A Retrospective GETTEC Multicenter Study
- PMID: 37445244
- PMCID: PMC10342877
- DOI: 10.3390/jcm12134210
Transoral Laser Microsurgery versus Robot-Assisted Surgery for Squamous Cell Carcinoma of the Tongue Base (Oncological and Functional Results)-A Retrospective GETTEC Multicenter Study
Abstract
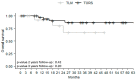
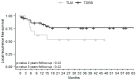
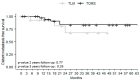
The base of the tongue (BOT) is the second most common site for squamous cell carcinoma (SCC) in the oropharynx. There are currently no clear guidelines for the management of BOT SCC. Our main objective was to compare the oncological outcomes of two minimally invasive approaches, transoral laser microsurgery (TLM) and transoral robot-assisted surgery (TORS). This was a retrospective French GETTEC (Groupe d'Études des Tumeurs de la Tête et du Cou) multicenter study of patients with BOT SCC removed surgically either by TLM or TORS between 2005 and 2021. The study group included 16 patients treated by TLM and 38 by TORS, with median follow-up times of 14.4 and 37.2 months, respectively. The overall survival (OS) rates at 2 and 3 years were 67% in the TLM group and 90% at 2 years and 86% at 3 years in the TORS group (p = 0.42, p = 0.20). There was no significant difference in recurrence-free survival (RFS) between the two techniques after 2 and 3 years. The tumors removed by TORS were significantly larger. Operative times were significantly shorter in the TLM group. There were no differences in feeding resumption; none of the patients in the TLM group required a tracheotomy. Postoperative hemorrhagic complication rates were similar in the two groups (12% for TLM and 13% for TORS). Both TORS and TLM showed encouraging oncological, functional, and safety results in BOT SCC even in recurrence or second primary cancer patients, without a technique being found superior in terms of OS or RFS. Tumors removed by TORS were larger without an increase in postoperative bleeding, extending the possibilities of transoral treatment.
Keywords: TLM; TORS; base of tongue; oropharynx squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. - DOI - PMC - PubMed
-
- O’Sullivan B., Huang S.H., Su J., Garden A.S., Sturgis E.M., Dahlstrom K., Lee N., Riaz N., Pei X., Koyfman S.A., et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. - DOI - PubMed
-
- Cracchiolo J.R., Baxi S.S., Morris L.G., Ganly I., Patel S.G., Cohen M.A., Roman B.R. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base: Increase in Primary Surgery for T1-T2 OPSCC. Cancer. 2016;122:1523–1532. doi: 10.1002/cncr.29938. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

