Hybrid Molecules of Benzylguanidine and the Alkylating Group of Melphalan: Synthesis and Effects on Neuroblastoma Cells
- PMID: 37445504
- PMCID: PMC10342853
- DOI: 10.3390/jcm12134469
Hybrid Molecules of Benzylguanidine and the Alkylating Group of Melphalan: Synthesis and Effects on Neuroblastoma Cells
Abstract
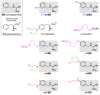
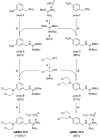
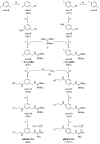
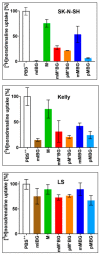
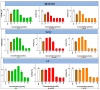
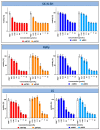
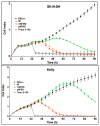
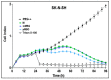
The therapy of neuroblastoma relies, amongst other things, on administering chemotherapeutics and radioactive compounds, e.g., the (meta-iodobenzyl)guanidine [131I]mIBG. For special applications (conditioning before stem cell transplantation), busulfan and melphalan (M) proved to be effective. However, both drugs are not used for normal chemotherapy in neuroblastoma because of their side effects. The alkylating drug melphalan contains a (Cl-CH2-CH2-)2N- group in the para-position of the phenyl moiety of the essential amino acid phenylalanine (Phe) and can, therefore, be taken up by virtually all kinds of cells by amino acid transporters. In contrast, mIBG isotopologs are taken up more selectively by neuroblastoma cells via the noradrenaline transporter (NAT). The present study aimed at synthesising and studying hybrid molecules of benzylguanidine (BG) and the alkylating motif of M. Such hybrids should combine the preferential uptake of BGs into neuroblastoma cells with the cytotoxicity of M. Besides the hybrid of BG with the dialkylating group (Cl-CH2-CH2-)2N- bound in the para-position as in M (pMBG), we also synthesised mMBG, which is BG meta-substituted by a (Cl-CH2-CH2-)2N- group. Furthermore, two monoalkylating hybrid molecules were synthesised: the BG para-substituted by a (Cl-CH2-CH2-)NH- group (pM*BG) and the BG meta-substituted by a (Cl-CH2-CH2-)NH- group (mM*BG). The effects of the four new compounds were studied with human neuroblastoma cell lines (SK-N-SH, Kelly, and LS) with regard to uptake, viability, and proliferation by standard test systems. The dialkylating hybrid molecules pMBG and mMBG were at least as effective as M, whereas the monoalkylating hybrid molecules pM*BG and mM*BG were more effective than M. Considering the preferred uptake via the noradrenaline transporter by neuroblastoma cells, we conclude that they might be well suited for therapy.
Keywords: (meta-iodobenzyl)guanidine (mIBG); melphalan (M); melphalan benzylguanidine hybrids (MBGs); neuroblastoma; noradrenaline transporter (NAT); organic cation transporter (OCT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis and biological effects of new hybrid compounds composed of benzylguanidines and the alkylating group of busulfan on neuroblastoma cells.Bioorg Med Chem Lett. 2014 Jun 15;24(12):2728-33. doi: 10.1016/j.bmcl.2014.04.030. Epub 2014 Apr 18. Bioorg Med Chem Lett. 2014. PMID: 24814532
-
Uptake of mIBG and catecholamines in noradrenaline- and organic cation transporter-expressing cells: potential use of corticosterone for a preferred uptake in neuroblastoma- and pheochromocytoma cells.Nucl Med Biol. 2009 Apr;36(3):287-94. doi: 10.1016/j.nucmedbio.2008.12.010. Nucl Med Biol. 2009. PMID: 19324274
-
Chemical characterization and comparative cellular effects of meta-iodobenzyl guanidine and benzyl guanidine.Cancer Chemother Pharmacol. 1997;40(2):131-7. doi: 10.1007/s002800050637. Cancer Chemother Pharmacol. 1997. PMID: 9182834
-
Potentiation of anti-cancer drug activity at low intratumoral pH induced by the mitochondrial inhibitor m-iodobenzylguanidine (MIBG) and its analogue benzylguanidine (BG).Br J Cancer. 1999 Feb;79(5-6):793-801. doi: 10.1038/sj.bjc.6690127. Br J Cancer. 1999. PMID: 10070871 Free PMC article.
-
A 4-methyl-substituted meta-iodobenzylguanidine analogue with prolonged retention in human neuroblastoma cells.Eur J Nucl Med Mol Imaging. 2004 Oct;31(10):1362-70. doi: 10.1007/s00259-004-1596-8. Epub 2004 Jun 16. Eur J Nucl Med Mol Imaging. 2004. PMID: 15205923
References
-
- Ray S.K., editor. Neuroblastoma. Molecular Mechanisms and Therapeutic Interventions. Elsevier; Amsterdam, The Netherlands: Academic Press; Cambridge, MA, USA: 2019.
-
- Abbas A.A., Samkari A.M.N. High-Risk Neuroblastoma: Poor outcomes despite aggressive multimodal therapy. Curr. Canc. Ther. Rev. 2022;18:14–40. doi: 10.2174/1573394717666210805114226. - DOI
-
- Glowniak J.V., Kilty J.E., Amara S.G., Hoffman B.J., Turner F.E. Evaluation of meta-iodobenzylguanidine uptake by the norepinephrine, dopamine and serotonine transporters. J. Nucl. Med. 1993;34:1140–1146. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

