IL-22 Is Deleterious along with IL-17 in Allergic Asthma but Is Not Detrimental in the Comorbidity Asthma and Acute Pneumonia
- PMID: 37445595
- PMCID: PMC10341917
- DOI: 10.3390/ijms241310418
IL-22 Is Deleterious along with IL-17 in Allergic Asthma but Is Not Detrimental in the Comorbidity Asthma and Acute Pneumonia
Abstract
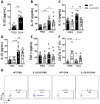
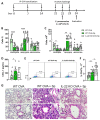
There is evidence that IL-22 and IL-17 participate in the pathogenesis of allergic asthma. To investigate the role of IL-22, we used IL-22 deficient mice (IL-22 KO) sensitized and challenged with ovalbumin (OVA) and compared with wild type (WT) animals exposed to OVA. IL-22 KO animals exposed to OVA showed a decreased number and frequency of eosinophils, IL-5 and IL-13 in the airways, reduced mucus production and pulmonary inflammation. In addition, IL-22 KO animals exhibited a decreased percentage and number of lung CD11c+CD11b+ cells and increased apoptosis of eosinophils. Th17 cell transfer generated from IL-22 KO to animals previously sensitized and challenged with OVA caused a reduction in eosinophil frequency and number in the airways compared to animals transferred with Th17 cells generated from WT mice. Therefore, IL-22 is deleterious with concomitant secretion of IL-17. Our findings show a pro-inflammatory role for IL-22, confirmed in a model of allergen-free and allergen-specific immunotherapy. Moreover, during the comorbidity asthma and pneumonia that induces neutrophil inflammation, IL-22 was not detrimental. Our results show that targeting IL-22 would negatively affect the survival of eosinophils, reduce the expansion or migration of CD11c+CD11b+ cells, and negatively regulate allergic asthma.
Keywords: IL-17; IL-22; apoptosis; asthma; dendritic cell; eosinophil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice.Am J Physiol Lung Cell Mol Physiol. 2012 Feb 15;302(4):L429-40. doi: 10.1152/ajplung.00252.2011. Epub 2011 Dec 16. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22180658
-
Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model.J Asthma. 2017 Jun;54(5):447-455. doi: 10.1080/02770903.2016.1223687. Epub 2016 Sep 2. J Asthma. 2017. PMID: 27589490
-
Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice.Phytomedicine. 2019 Aug;61:152835. doi: 10.1016/j.phymed.2019.152835. Epub 2019 Jan 16. Phytomedicine. 2019. PMID: 31035047
-
Protective Role of Eosinophils and TNFa after Ozone Inhalation.Res Rep Health Eff Inst. 2017 Mar;2017(191):1-41. Res Rep Health Eff Inst. 2017. PMID: 29659241 Free PMC article.
-
Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma.Respir Res. 2014 Jun 7;15(1):63. doi: 10.1186/1465-9921-15-63. Respir Res. 2014. PMID: 24907978 Free PMC article.
Cited by
-
Advances in understanding the role of interleukins in pulmonary fibrosis (Review).Exp Ther Med. 2024 Nov 28;29(2):25. doi: 10.3892/etm.2024.12775. eCollection 2025 Feb. Exp Ther Med. 2024. PMID: 39650776 Free PMC article. Review.
References
-
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention 2022 Update. Glob. Initiat. Asthma. 2022;225
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

