An Efficient 5-Aminolevulinic Acid Photodynamic Therapy Treatment for Human Hepatocellular Carcinoma
- PMID: 37445603
- PMCID: PMC10341487
- DOI: 10.3390/ijms241310426
An Efficient 5-Aminolevulinic Acid Photodynamic Therapy Treatment for Human Hepatocellular Carcinoma
Abstract
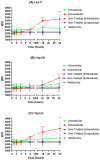
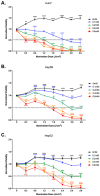
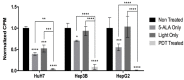
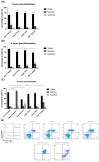
Photodynamic therapy (PDT) is a two-stage treatment relying on cytotoxicity induced by photoexcitation of a nontoxic dye, called photosensitizer (PS). Using 5-aminolevulinic acid (5-ALA), the pro-drug of PS protoporphyrin IX, we investigated the impact of PDT on hepatocellular carcinoma (HCC). Optimal 5-ALA PDT dose was determined on three HCC cell lines by analyzing cell death after treatment with varying doses. HCC-patient-derived tumor hepatocytes and healthy donor liver myofibroblasts were treated with optimal 5-ALA PDT doses. The proliferation of cancer cells and healthy donor immune cells cultured with 5-ALA-PDT-treated conditioned media was analyzed. Finally, therapy efficacy on humanized SCID mice model of HCC was investigated. 5-ALA PDT induced a dose-dependent decrease in viability, with an up-to-four-fold reduction in viability of patient tumor hepatocytes. The 5-ALA PDT treated conditioned media induced immune cell clonal expansion. 5-ALA PDT has no impact on myofibroblasts in terms of viability, while their activation decreased cancer cell proliferation and reduced the tumor growth rate of the in vivo model. For the first time, 5-ALA PDT has been validated on primary patient tumor hepatocytes and donor healthy liver myofibroblasts. 5-ALA PDT may be an effective anti-HCC therapy, which might induce an anti-tumor immune response.
Keywords: 5-ALA; anti-tumor immunity; anti-tumor immunotherapy; hepatocellular carcinoma; photodynamic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

