Cross-Kingdom Interaction of miRNAs and Gut Microbiota with Non-Invasive Diagnostic and Therapeutic Implications in Colorectal Cancer
- PMID: 37445698
- PMCID: PMC10341587
- DOI: 10.3390/ijms241310520
Cross-Kingdom Interaction of miRNAs and Gut Microbiota with Non-Invasive Diagnostic and Therapeutic Implications in Colorectal Cancer
Abstract
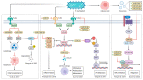
Colorectal cancer (CRC) has one of the highest incidences among all types of malignant diseases, affecting millions of people worldwide. It shows slow progression, making it preventable. However, this is not the case due to shortcomings in its diagnostic and management procedure and a lack of effective non-invasive biomarkers for screening. Here, we discuss CRC-associated microRNAs (miRNAs) and gut microbial species with potential as CRC diagnostic and therapy biomarkers. We provide rich evidence of cross-kingdom miRNA-mediated interactions between the host and gut microbiome. miRNAs have emerged with the ability to shape the composition and dynamics of gut microbiota. Intestinal microbes can uptake miRNAs, which in turn influence microbial growth and provide the ability to regulate the abundance of various microbial species. In the context of CRC, targeting miRNAs could aid in manipulating the balance of the microbiota. Our findings suggest the need for correlation analysis between the composition of the gut microbiome and the miRNA expression profile.
Keywords: biomarkers; colorectal cancer; dysbiosis; gut microbiota; microRNAs.
Conflict of interest statement
The authors G.B., M.Z., L.L., K.B., E.H.T., T.R., A.C., V.R., and B.N. declare no conflicts of interest that could influence the work reported in this paper. O.P., J.S., and T.S. are employees of Geneton Ltd. and are involved in numerous research and development efforts for adapting new technologies to better understand genomic data and facilitate their implementation in patient care.
Figures

Similar articles
-
Host⁻MicroRNA⁻Microbiota Interactions in Colorectal Cancer.Genes (Basel). 2019 Apr 2;10(4):270. doi: 10.3390/genes10040270. Genes (Basel). 2019. PMID: 30987065 Free PMC article. Review.
-
Interaction between Host MicroRNAs and the Gut Microbiota in Colorectal Cancer.mSystems. 2018 May 15;3(3):e00205-17. doi: 10.1128/mSystems.00205-17. eCollection 2018 May-Jun. mSystems. 2018. PMID: 29795787 Free PMC article.
-
miRNA-Microbiota Interaction in Gut Homeostasis and Colorectal Cancer.Trends Cancer. 2019 Nov;5(11):666-669. doi: 10.1016/j.trecan.2019.08.003. Epub 2019 Oct 14. Trends Cancer. 2019. PMID: 31735285 Free PMC article. Review.
-
Gut Microbiome in Colorectal Cancer: Clinical Diagnosis and Treatment.Genomics Proteomics Bioinformatics. 2023 Feb;21(1):84-96. doi: 10.1016/j.gpb.2022.07.002. Epub 2022 Jul 30. Genomics Proteomics Bioinformatics. 2023. PMID: 35914737 Free PMC article. Review.
-
Meta-Analysis of Altered Gut Microbiota Reveals Microbial and Metabolic Biomarkers for Colorectal Cancer.Microbiol Spectr. 2022 Aug 31;10(4):e0001322. doi: 10.1128/spectrum.00013-22. Epub 2022 Jun 29. Microbiol Spectr. 2022. PMID: 35766483 Free PMC article.
Cited by
-
Serum Cytokine and miRNA Levels Are Differently Expressed in Right- and Left-Sided Colon Cancer.J Clin Med. 2023 Sep 15;12(18):5986. doi: 10.3390/jcm12185986. J Clin Med. 2023. PMID: 37762927 Free PMC article.
References
-
- Halloran S.P., Launoy G., Zappa M. International Agency for Research on Cancer European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. First Edition—Faecal Occult Blood Testing. Endoscopy. 2012;44((Suppl. S3)):SE65–SE87. - PubMed
-
- US Preventive Services Task Force. Davidson K.W., Barry M.J., Mangione C.M., Cabana M., Caughey A.B., Davis E.M., Donahue K.E., Doubeni C.A., Krist A.H., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965–1977. - PubMed
-
- de Klaver W., Wisse P.H.A., van Wifferen F., Bosch L.J.W., Jimenez C.R., van der Hulst R.W.M., Fijneman R.J.A., Kuipers E.J., Greuter M.J.E., Carvalho B., et al. Clinical Validation of a Multitarget Fecal Immunochemical Test for Colorectal Cancer Screening: A Diagnostic Test Accuracy Study. Ann. Intern. Med. 2021;174:1224–1231. doi: 10.7326/M20-8270. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

