Secretogranin III Selectively Promotes Vascular Leakage in the Deep Vascular Plexus of Diabetic Retinopathy
- PMID: 37445707
- PMCID: PMC10341987
- DOI: 10.3390/ijms241310531
Secretogranin III Selectively Promotes Vascular Leakage in the Deep Vascular Plexus of Diabetic Retinopathy
Abstract
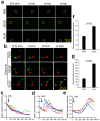
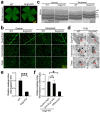
Diabetic retinopathy (DR), a leading cause of vision loss in working-age adults, induces mosaic patterns of vasculopathy that may be associated with spatial heterogeneity of intraretinal endothelial cells. We recently reported that secretogranin III (Scg3), a neuron-derived angiogenic and vascular leakage factor, selectively binds retinal vessels of diabetic but not healthy mice. Here, we investigated endothelial heterogeneity of three retinal vascular plexuses in DR pathogenesis and the therapeutic implications. Our unique in vivo ligand binding assay detected a 22.7-fold increase in Scg3 binding to retinal vessels of diabetic mice relative to healthy mice. Functional immunohistochemistry revealed that Scg3 predominantly binds to the DR-stressed CD31- deep retinal vascular plexus but not to the relatively healthy CD31+ superficial and intermediate plexuses within the same diabetic retina. In contrast, VEGF bound to healthy and diabetic retinal vessels indiscriminately with low activity. FITC-dextran assays indicated that selectively increased retinal vascular leakage coincides with Scg3 binding in diabetic mice that was independent of VEGF, whereas VEGF-induced leakage did not distinguish between diabetic and healthy mice. Dose-response curves showed that the anti-Scg3 humanized antibody (hAb) and anti-VEGF aflibercept alleviated DR leakage with equivalent efficacies, and that the combination acted synergistically. These findings suggest: (i) the deep plexus is highly sensitive to DR; (ii) Scg3 binding to the DR deep plexus coincides with the loss of CD31 and compromised endothelial junctions; (iii) anti-Scg3 hAb alleviates vascular leakage by selectively targeting the DR-stressed deep plexus within the same diabetic retina; (iv) combined anti-Scg3 and anti-VEGF treatments synergistically ameliorate DR through distinct mechanisms.
Keywords: Scg3; VEGF; diabetic retinopathy; disease-targeted anti-angiogenic therapy; endothelial heterogeneity; synergistic combination anti-angiogenic therapy.
Conflict of interest statement
H.T. and W.L. are shareholders of Everglades Biopharma, LLC and LigandomicsRx, LLC. W.L. is an inventor of issued and pending patents. The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Neurovascular regulation in diabetic retinopathy and emerging therapies.Cell Mol Life Sci. 2021 Aug;78(16):5977-5985. doi: 10.1007/s00018-021-03893-9. Epub 2021 Jul 7. Cell Mol Life Sci. 2021. PMID: 34230991 Free PMC article. Review.
-
Secretogranin III as a disease-associated ligand for antiangiogenic therapy of diabetic retinopathy.J Exp Med. 2017 Apr 3;214(4):1029-1047. doi: 10.1084/jem.20161802. Epub 2017 Mar 22. J Exp Med. 2017. PMID: 28330905 Free PMC article.
-
Selectively targeting disease-restricted secretogranin III to alleviate choroidal neovascularization.FASEB J. 2022 Jan;36(1):e22106. doi: 10.1096/fj.202101085RR. FASEB J. 2022. PMID: 34918375 Free PMC article.
-
Optimal Humanized Scg3-Neutralizing Antibodies for Anti-Angiogenic Therapy of Diabetic Retinopathy.Int J Mol Sci. 2024 Sep 1;25(17):9507. doi: 10.3390/ijms25179507. Int J Mol Sci. 2024. PMID: 39273454 Free PMC article.
-
Secretogranin III: a diabetic retinopathy-selective angiogenic factor.Cell Mol Life Sci. 2018 Feb;75(4):635-647. doi: 10.1007/s00018-017-2635-5. Epub 2017 Aug 30. Cell Mol Life Sci. 2018. PMID: 28856381 Free PMC article. Review.
Cited by
-
Interleukin-4 prevents increased endothelial permeability by inducing pericyte survival and modulating microglial responses in diabetic retinopathy.Front Endocrinol (Lausanne). 2025 Jul 2;16:1609796. doi: 10.3389/fendo.2025.1609796. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40671907 Free PMC article.
-
Mendelian Randomization Estimates the Effects of Plasma and Cerebrospinal Fluid Proteins on Intelligence, Fluid Intelligence Score, and Cognitive Performance.Mol Neurobiol. 2025 Apr;62(4):4922-4934. doi: 10.1007/s12035-024-04542-5. Epub 2024 Nov 4. Mol Neurobiol. 2025. PMID: 39495227
-
A method for rapid and reliable quantification of VEGF-cell binding activity.Biochem Biophys Res Commun. 2024 Oct 1;727:150321. doi: 10.1016/j.bbrc.2024.150321. Epub 2024 Jun 27. Biochem Biophys Res Commun. 2024. PMID: 38954982
-
Suppression of matrigel-induced choroidal neovascularization by AAV delivery of a novel anti-Scg3 antibody.Gene Ther. 2024 Nov;31(11-12):587-593. doi: 10.1038/s41434-024-00491-9. Epub 2024 Sep 27. Gene Ther. 2024. PMID: 39333408
-
Secretogranin III: a promising therapeutic target for intraocular neovascular lesions.Int Ophthalmol. 2025 Jan 20;45(1):26. doi: 10.1007/s10792-024-03393-2. Int Ophthalmol. 2025. PMID: 39832055 Free PMC article. Review.
References
-
- Teo Z.L., Tham Y.-C., Yu M., Chee M.L., Rim T.H., Cheung N., Bikbov M.M., Wang Y.X., Tang Y., Lu Y., et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580–1591. doi: 10.1016/j.ophtha.2021.04.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

