Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles
- PMID: 37445712
- PMCID: PMC10341914
- DOI: 10.3390/ijms241310536
Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles
Abstract
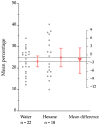
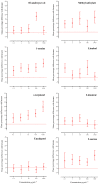
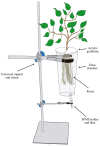
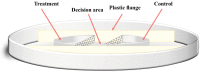
The foraging behavior of the infective juveniles (IJs) of entomopathogenic nematodes (EPNs) relies on host-derived compounds, but in a tri-trophic context, herbivore-induced root volatiles act as signals enhancing the biological control of insect pests by recruiting EPNs. In southern Chile, the EPN Steinernema australe exhibits the potential to control the raspberry weevil, Aegorhinus superciliosus, a key pest of blueberry Vaccinium corymbosum. However, there is no information on the quality of the blueberry root volatile plume or the S. australe response to these chemicals as putative attractants. Here, we describe the root volatile profile of blueberries and the chemotaxis behavior of S. australe towards the volatiles identified from Vaccinium corymbosum roots, infested or uninfested with A. superciliosus larvae. Among others, we found linalool, α-terpineol, limonene, eucalyptol, 2-carene, 1-nonine, 10-undecyn-1-ol, and methyl salicylate in root volatiles and, depending on the level of the emissions, they were selected for bioassays. In the dose-response tests, S. australe was attracted to all five tested concentrations of methyl salicylate, 1-nonine, α-terpineol, and 2-carene, as well as to 100 µg mL-1 of 10-undecyn-1-ol, 0.1 and 100 µg mL-1 of linalool, and 100 µg mL-1 of limonene, whereas eucalyptol elicited no attraction or repellency. These results suggest that some volatiles released from damaged roots attract S. australe and may have implications for the biocontrol of subterranean pests.
Keywords: belowground interactions; entomopathogenic nematode recruitment; foraging behavior; olfactometer; root volatiles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Volatiles released from Vaccinium corymbosum were attractive to Aegorhinus superciliosus (Coleoptera: Curculionidae) in an olfactometric bioassay.Environ Entomol. 2009 Jun;38(3):781-9. doi: 10.1603/022.038.0330. Environ Entomol. 2009. PMID: 19508787
-
Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes.J Chem Ecol. 2010 Apr;36(4):361-8. doi: 10.1007/s10886-010-9773-7. Epub 2010 Mar 23. J Chem Ecol. 2010. PMID: 20309617
-
Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats.PLoS One. 2012;7(6):e38146. doi: 10.1371/journal.pone.0038146. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761668 Free PMC article.
-
Entomopathogenic nematodes, root weevil larvae, and dynamic interactions among soil texture, plant growth, herbivory, and predation.J Invertebr Pathol. 2012 Jan;109(1):134-42. doi: 10.1016/j.jip.2011.10.012. Epub 2011 Oct 29. J Invertebr Pathol. 2012. PMID: 22056274
-
Insect pathogens as biological control agents: Back to the future.J Invertebr Pathol. 2015 Nov;132:1-41. doi: 10.1016/j.jip.2015.07.009. Epub 2015 Jul 27. J Invertebr Pathol. 2015. PMID: 26225455 Review.
Cited by
-
Phylum Level Diversity of Plant Interior Bacteria in Seeds, Supernatant and Pellet Phases of Seed Suspension of Mustard Plant.Indian J Microbiol. 2024 Dec;64(4):1587-1597. doi: 10.1007/s12088-023-01184-4. Epub 2024 Feb 10. Indian J Microbiol. 2024. PMID: 39678952
-
Entomopathogenic Nematode Species Vary in Their Behavior and Virulence in Response to Cardiac Glycosides Within and Around Insect Hosts.J Chem Ecol. 2025 Jan 27;51(1):12. doi: 10.1007/s10886-025-01563-9. J Chem Ecol. 2025. PMID: 39869279 Free PMC article.
References
-
- Rasmann S., Erwin A.C., Halitschke R., Agrawal A.A. Direct and indirect root defences of milkweed (Asclepias syriaca): Trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J. Ecol. 2011;99:16–25. doi: 10.1111/j.1365-2745.2010.01713.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

