Enhanced ZBTB16 Levels by Progestin-Only Contraceptives Induces Decidualization and Inflammation
- PMID: 37445713
- PMCID: PMC10341894
- DOI: 10.3390/ijms241310532
Enhanced ZBTB16 Levels by Progestin-Only Contraceptives Induces Decidualization and Inflammation
Abstract
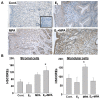
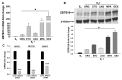
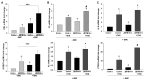
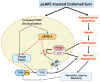
Progestin-only long-acting reversible-contraceptive (pLARC)-exposed endometria displays decidualized human endometrial stromal cells (HESCs) and hyperdilated thin-walled fragile microvessels. The combination of fragile microvessels and enhanced tissue factor levels in decidualized HESCs generates excess thrombin, which contributes to abnormal uterine bleeding (AUB) by inducing inflammation, aberrant angiogenesis, and proteolysis. The- zinc finger and BTB domain containing 16 (ZBTB16) has been reported as an essential regulator of decidualization. Microarray studies have demonstrated that ZBTB16 levels are induced by medroxyprogesterone acetate (MPA) and etonogestrel (ETO) in cultured HESCs. We hypothesized that pLARC-induced ZBTB16 expression contributes to HESC decidualization, whereas prolonged enhancement of ZBTB16 levels triggers an inflammatory milieu by inducing pro-inflammatory gene expression and tissue-factor-mediated thrombin generation in decidualized HESCs. Thus, ZBTB16 immunostaining was performed in paired endometria from pre- and post-depo-MPA (DMPA)-administrated women and oophorectomized guinea pigs exposed to the vehicle, estradiol (E2), MPA, or E2 + MPA. The effect of progestins including MPA, ETO, and levonorgestrel (LNG) and estradiol + MPA + cyclic-AMP (E2 + MPA + cAMP) on ZBTB16 levels were measured in HESC cultures by qPCR and immunoblotting. The regulation of ZBTB16 levels by MPA was evaluated in glucocorticoid-receptor-silenced HESC cultures. ZBTB16 was overexpressed in cultured HESCs for 72 h followed by a ± 1 IU/mL thrombin treatment for 6 h. DMPA administration in women and MPA treatment in guinea pigs enhanced ZBTB16 immunostaining in endometrial stromal and glandular epithelial cells. The in vitro findings indicated that: (1) ZBTB16 levels were significantly elevated by all progestin treatments; (2) MPA exerted the greatest effect on ZBTB16 levels; (3) MPA-induced ZBTB16 expression was inhibited in glucocorticoid-receptor-silenced HESCs. Moreover, ZBTB16 overexpression in HESCs significantly enhanced prolactin (PRL), insulin-like growth factor binding protein 1 (IGFBP1), and tissue factor (F3) levels. Thrombin-induced interleukin 8 (IL-8) and prostaglandin-endoperoxide synthase 2 (PTGS2) mRNA levels in control-vector-transfected HESCs were further increased by ZBTB16 overexpression. In conclusion, these results supported that ZBTB16 is enhanced during decidualization, and long-term induction of ZBTB16 expression by pLARCs contributes to thrombin generation through enhancing tissue factor expression and inflammation by enhancing IL-8 and PTGS2 levels in decidualized HESCs.
Keywords: COX-2; IL-8; ZBTB16; decidualization; endometrial stromal cells; progestin-only contraceptives; tissue factor.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures


Similar articles
-
Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding.J Clin Endocrinol Metab. 2004 Mar;89(3):1467-75. doi: 10.1210/jc.2003-030141. J Clin Endocrinol Metab. 2004. PMID: 15001649
-
Progestins Upregulate FKBP51 Expression in Human Endometrial Stromal Cells to Induce Functional Progesterone and Glucocorticoid Withdrawal: Implications for Contraceptive- Associated Abnormal Uterine Bleeding.PLoS One. 2015 Oct 5;10(10):e0137855. doi: 10.1371/journal.pone.0137855. eCollection 2015. PLoS One. 2015. PMID: 26436918 Free PMC article.
-
Progestin-regulated expression of tissue factor in decidual cells: implications in endometrial hemostasis, menstruation and angiogenesis.Steroids. 2003 Nov;68(10-13):849-60. doi: 10.1016/s0039-128x(03)00139-9. Steroids. 2003. PMID: 14667977 Review.
-
Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell stromelysin-1 and prolactin expression.J Clin Endocrinol Metab. 1997 Jan;82(1):188-93. doi: 10.1210/jcem.82.1.3677. J Clin Endocrinol Metab. 1997. PMID: 8989257
-
The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding.Hum Reprod Update. 2016 Jun;22(4):497-515. doi: 10.1093/humupd/dmw004. Epub 2016 Feb 23. Hum Reprod Update. 2016. PMID: 26912000 Free PMC article. Review.
Cited by
-
Membrane Progesterone Receptor Beta Regulates the Decidualization of Endometrial Stromal Cells in Women with Endometriosis.Int J Mol Sci. 2025 Jul 28;26(15):7297. doi: 10.3390/ijms26157297. Int J Mol Sci. 2025. PMID: 40806428 Free PMC article.
-
The glucocorticoid receptor is affected by its target ZBTB16 in a dissociated manner.J Endocrinol. 2025 Jul 4;266(1):e240283. doi: 10.1530/JOE-24-0283. Print 2025 Jul 1. J Endocrinol. 2025. PMID: 40540650 Free PMC article.
-
Establishment of Murine Pregnancy Requires the Promyelocytic Leukemia Zinc Finger Transcription Factor.Int J Mol Sci. 2024 Mar 19;25(6):3451. doi: 10.3390/ijms25063451. Int J Mol Sci. 2024. PMID: 38542422 Free PMC article.
References
-
- Haddad L.B., Herring G.B., Mehta C.C., Staple T., Young M.R., Govindaraj S., Velu V., Smith A.K. Evaluating the impact of three progestin-based hormonal contraceptive methods on immunologic changes in the female genital tract and systemically (CHIME Study): A prospective cohort study protocol. BMC Womens Health. 2022;22:456. doi: 10.1186/s12905-022-02053-w. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

