Rotenone Blocks the Glucocerebrosidase Enzyme and Induces the Accumulation of Lysosomes and Autophagolysosomes Independently of LRRK2 Kinase in HEK-293 Cells
- PMID: 37445771
- PMCID: PMC10341970
- DOI: 10.3390/ijms241310589
Rotenone Blocks the Glucocerebrosidase Enzyme and Induces the Accumulation of Lysosomes and Autophagolysosomes Independently of LRRK2 Kinase in HEK-293 Cells
Abstract
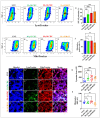
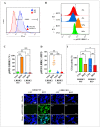
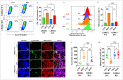
Parkinson's disease (PD) is a neurodegenerative disorder caused by the progressive loss of dopaminergic (DAergic) neurons in the substantia nigra and the intraneuronal presence of Lewy bodies (LBs), composed of aggregates of phosphorylated alpha-synuclein at residue Ser129 (p-Ser129α-Syn). Unfortunately, no curative treatment is available yet. To aggravate matters further, the etiopathogenesis of the disorder is still unresolved. However, the neurotoxin rotenone (ROT) has been implicated in PD. Therefore, it has been widely used to understand the molecular mechanism of neuronal cell death. In the present investigation, we show that ROT induces two convergent pathways in HEK-293 cells. First, ROT generates H2O2, which, in turn, either oxidizes the stress sensor protein DJ-Cys106-SH into DJ-1Cys106SO3 or induces the phosphorylation of the protein LRRK2 kinase at residue Ser395 (p-Ser395 LRRK2). Once active, the kinase phosphorylates α-Syn (at Ser129), induces the loss of mitochondrial membrane potential (ΔΨm), and triggers the production of cleaved caspase 3 (CC3), resulting in signs of apoptotic cell death. ROT also reduces glucocerebrosidase (GCase) activity concomitant with the accumulation of lysosomes and autophagolysosomes reflected by the increase in LC3-II (microtubule-associated protein 1A/1B-light chain 3-phosphatidylethanolamine conjugate II) markers in HEK-293 cells. Second, the exposure of HEK-293 LRRK2 knockout (KO) cells to ROT displays an almost-normal phenotype. Indeed, KO cells showed neither H2O2, DJ-1Cys106SO3, p-Ser395 LRRK2, p-Ser129α-Syn, nor CC3 but displayed high ΔΨm, reduced GCase activity, and the accumulation of lysosomes and autophagolysosomes. Similar observations are obtained when HEK-293 LRRK2 wild-type (WT) cells are exposed to the inhibitor GCase conduritol-β-epoxide (CBE). Taken together, these observations imply that the combined development of LRRK2 inhibitors and compounds for recovering GCase activity might be promising therapeutic agents for PD.
Keywords: HEK-293; LRRK2; Parkinson’s; autophagy; conduritol-β-epoxide; glucocerebrosidase; rotenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

