Microbial Metabolites of 3- n-butylphthalide as Monoamine Oxidase A Inhibitors
- PMID: 37445788
- PMCID: PMC10342118
- DOI: 10.3390/ijms241310605
Microbial Metabolites of 3- n-butylphthalide as Monoamine Oxidase A Inhibitors
Abstract
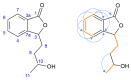
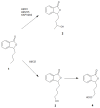
Novel compounds with antidepressant activity via monoamine oxidase inhibition are being sought. Among these, derivatives of 3-n-butylphthalide, a neuroprotective lactone from Apiaceae plants, may be prominent candidates. This study aimed to obtain the oxidation products of 3-n-butylphthalide and screen them regarding their activity against the monoamine oxidase A (MAO-A) isoform. Such activity of these compounds has not been previously tested. To obtain the metabolites, we used fungi as biocatalysts because of their high oxidative capacity. Overall, 37 strains were used, among which Penicillium and Botrytis spp. were the most efficient, leading to the obtaining of three main products: 3-n-butyl-10-hydroxyphthalide, 3-n-butylphthalide-11-oic acid, and 3-n-butyl-11-hydroxyphthalide, with a total yield of 0.38-0.82 g per g of the substrate, depending on the biocatalyst used. The precursor-3-n-butylphthalide and abovementioned metabolites inhibited the MAO-A enzyme; the most active was the carboxylic acid derivative of the lactone with inhibitory constant (Ki) < 0.001 µmol/L. The in silico prediction of the drug-likeness of the metabolites matches the assumptions of Lipinski, Ghose, Veber, Egan, and Muegge. All the compounds are within the optimal range for the lipophilicity value, which is connected to adequate permeability and solubility.
Keywords: 3-n-butylphthalide; biotransformation; fungal strains; monoamine oxidase A inhibitor; serotonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
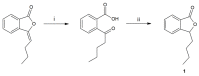


Similar articles
-
[Effect of various antidepressive agents on brain monoamine oxidase activity].Biull Eksp Biol Med. 1982 Nov;94(11):29-31. Biull Eksp Biol Med. 1982. PMID: 7150732 Russian.
-
Potent Selective Inhibition of Monoamine Oxidase A by Alternariol Monomethyl Ether Isolated from Alternaria brassicae.J Microbiol Biotechnol. 2017 Feb 28;27(2):316-320. doi: 10.4014/jmb.1610.10053. J Microbiol Biotechnol. 2017. PMID: 27840401
-
Attenuation of the effects of oxidative stress by the MAO-inhibiting antidepressant and carbonyl scavenger phenelzine.Chem Biol Interact. 2019 May 1;304:139-147. doi: 10.1016/j.cbi.2019.03.003. Epub 2019 Mar 8. Chem Biol Interact. 2019. PMID: 30857888 Review.
-
Synthesis and Evaluation of 2-benzylidene-1-tetralone Derivatives for Monoamine Oxidase Inhibitory Activity.Cent Nerv Syst Agents Med Chem. 2018;18(2):136-149. doi: 10.2174/1871524918666180501121638. Cent Nerv Syst Agents Med Chem. 2018. PMID: 29714148
-
Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation.Neurotoxicology. 2004 Jan;25(1-2):243-50. doi: 10.1016/S0161-813X(03)00103-7. Neurotoxicology. 2004. PMID: 14697899 Review.
Cited by
-
Differentiating Tangerine Peels from Other Citrus reticulata through GC-MS, UPLC-Q-Exactive Orbitrap-MS, and HPLC-PDA.ACS Omega. 2024 Dec 27;10(1):1688-1704. doi: 10.1021/acsomega.4c09701. eCollection 2025 Jan 14. ACS Omega. 2024. PMID: 39829587 Free PMC article.
References
-
- Greener M. New Antidepressants: Monoamines and Beyond. Prescriber. 2023;34:17–20. doi: 10.1002/psb.2032. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

