ECM Composition Differentially Regulates Intracellular and Extracellular pH in Normal and Cancer Pancreatic Duct Epithelial Cells
- PMID: 37445810
- PMCID: PMC10341693
- DOI: 10.3390/ijms241310632
ECM Composition Differentially Regulates Intracellular and Extracellular pH in Normal and Cancer Pancreatic Duct Epithelial Cells
Abstract
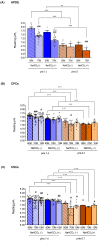
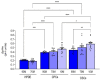
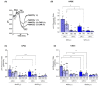
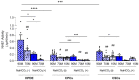
Intracellular pH (pHi) regulation is a challenge for the exocrine pancreas, where the luminal secretion of bicarbonate-rich fluid is accompanied by interstitial flows of acid. This acid-base transport requires a plethora of ion transporters, including bicarbonate transporters and the Na+/H+ exchanger isoform 1 (NHE1), which are dysregulated in Pancreatic Ductal Adenocarcinoma (PDAC). PDAC progression is favored by a Collagen-I rich extracellular matrix (ECM) which exacerbates the physiological interstitial acidosis. In organotypic cultures of normal human pancreatic cells (HPDE), parenchymal cancer cells (CPCs) and cancer stem cells (CSCs) growing on matrices reproducing ECM changes during progression, we studied resting pHi, the pHi response to fluxes of NaHCO3 and acidosis and the role of NHE1 in pHi regulation. Our findings show that: (i) on the physiological ECM, HPDE cells have the most alkaline pHi, followed by CSCs and CPCs, while a Collagen I-rich ECM reverses the acid-base balance in cancer cells compared to normal cells; (ii) both resting pHi and pHi recovery from an acid load are reduced by extracellular NaHCO3, especially in HPDE cells on a normal ECM; (iii) cancer cell NHE1 activity is less affected by NaHCO3. We conclude that ECM composition and the fluctuations of pHe cooperate to predispose pHi homeostasis towards the presence of NaHCO3 gradients similar to that expected in the tumor.
Keywords: NHE1; PDAC; bicarbonate transport.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Stark A., Eibl G. Pancreatic Ductal Adenocarcinoma. Pancreapedia: The Exocrine Pancreas Knowledge Base. 2015. [(accessed on 23 May 2015)]. Available online: https://pancreapedia.org/?q=node/9002.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

