Anti-Inflammatory Activity of Black Soldier Fly Oil Associated with Modulation of TLR Signaling: A Metabolomic Approach
- PMID: 37445812
- PMCID: PMC10341857
- DOI: 10.3390/ijms241310634
Anti-Inflammatory Activity of Black Soldier Fly Oil Associated with Modulation of TLR Signaling: A Metabolomic Approach
Abstract
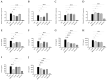
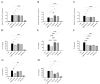
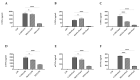
Dietary intervention in the treatment of ulcerative colitis involves, among other things, modifications in fatty acid content and/or profile. For example, replacing saturated long chain fatty acids with medium chain fatty acids (MCFAs) has been reported to ameliorate inflammation. The Black Soldier Fly Larvae's (BSFL) oil is considered a sustainable dietary ingredient rich in the MCFA C12:0; however, its effect on inflammatory-related conditions has not been studied until now. Thus, the present study aimed to investigate the anti-inflammatory activity of BSFL oil in comparison to C12:0 using TLR4- or TLR2-activated THP-1 and J774A.1 cell lines and to assess its putative protective effect against dextran sulfate sodium (DSS)-induced acute colitis in mice. BSFL oil and C12:0 suppressed proinflammatory cytokines release in LPS-stimulated macrophages; however, only BSFL oil exerted anti-inflammatory activity in Pam3CSK4-stimulated macrophages. Transcriptome analysis provided insight into the possible role of BSFL oil in immunometabolism switch, involving mTOR signaling and an increase in PPAR target genes promoting fatty acid oxidation, exhibiting a discrepant mode of action compared to C12:0 treatment, which mainly affected cholesterol biosynthesis pathways. Additionally, we identified anti-inflammatory eicosanoids, oxylipins, and isoprenoids in the BSFL oil that may contribute to an orchestrated anti-inflammatory response. In vivo, a BSFL oil-enriched diet (20%) ameliorated the clinical signs of colitis, as indicated by improved body weight recovery, reduced colon shortening, reduced splenomegaly, and an earlier phase of secretory IgA response. These results indicate the novel beneficial use of BSFL oil as a modulator of inflammation.
Keywords: Pam3CSK4; Toll-like receptor (TLR); black soldier fly larvae (BSFL); dextran sulfate sodium (DSS)-induced colitis; lipopolysaccharides (LPS); macrophage; mammalian target of rapamycin (mTOR); medium chain fatty acid (MCFA) C12:0; peroxisome proliferator-activated receptor (PPAR); proinflammatory cytokine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes.Int J Mol Sci. 2025 May 19;26(10):4855. doi: 10.3390/ijms26104855. Int J Mol Sci. 2025. PMID: 40429995 Free PMC article.
-
Black soldier fly larvae oil as an alternative fat source in broiler nutrition.Poult Sci. 2020 Jun;99(6):3133-3143. doi: 10.1016/j.psj.2020.01.018. Epub 2020 Mar 13. Poult Sci. 2020. PMID: 32475450 Free PMC article.
-
The impact of dietary Black Soldier Fly larvae oil and meal on laying hen performance and egg quality.Poult Sci. 2021 Aug;100(8):101272. doi: 10.1016/j.psj.2021.101272. Epub 2021 May 21. Poult Sci. 2021. PMID: 34237547 Free PMC article.
-
Black soldier fly (Hermetia illucens) larvae as potential feedstock for the biodiesel production: Recent advances and challenges.Sci Total Environ. 2023 Feb 10;859(Pt 1):160235. doi: 10.1016/j.scitotenv.2022.160235. Epub 2022 Nov 17. Sci Total Environ. 2023. PMID: 36402342 Review.
-
A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources.Environ Pollut. 2020 Dec;267:115488. doi: 10.1016/j.envpol.2020.115488. Epub 2020 Aug 26. Environ Pollut. 2020. PMID: 32891050 Review.
Cited by
-
Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes.Int J Mol Sci. 2025 May 19;26(10):4855. doi: 10.3390/ijms26104855. Int J Mol Sci. 2025. PMID: 40429995 Free PMC article.
-
Fatty acids and lipid mediators in inflammatory bowel disease: from mechanism to treatment.Front Immunol. 2023 Oct 5;14:1286667. doi: 10.3389/fimmu.2023.1286667. eCollection 2023. Front Immunol. 2023. PMID: 37868958 Free PMC article. Review.
-
The Effect of Dietary Protein Hydrolysate from Black Soldier Fly Larvae and Schizochytrium on Palatability, Nutrient Metabolites and Health Status in Beagle Dogs.Metabolites. 2024 Mar 14;14(3):165. doi: 10.3390/metabo14030165. Metabolites. 2024. PMID: 38535325 Free PMC article.
-
Using gut microbiota and non-targeted metabolomics techniques to study the effect of xylitol on alleviating DSS-induced inflammatory bowel disease in mice.BMC Immunol. 2025 Mar 10;26(1):18. doi: 10.1186/s12865-025-00700-z. BMC Immunol. 2025. PMID: 40065221 Free PMC article.
-
Hermetia illucens oil vs. hydrogenated palm fat in dairy cow nutrition: effects on digestive parameters, oxidative stress, and milk production performance.J Anim Sci Biotechnol. 2025 Jun 26;16(1):90. doi: 10.1186/s40104-025-01222-9. J Anim Sci Biotechnol. 2025. PMID: 40563124 Free PMC article.
References
-
- Lancaster G.I., Langley K.G., Berglund N.A., Kammoun H.L., Reibe S., Estevez E., Weir J., Mellett N.A., Pernes G., Conway J.R.W., et al. Evidence That TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018;27:1096–1110.e5. doi: 10.1016/j.cmet.2018.03.014. - DOI - PubMed
-
- Nicholas D.A., Zhang K., Hung C., Glasgow S., Aruni A.W., Unternaehrer J., Payne K.J., Langridge W.H.R., De Leon M. Palmitic Acid Is a Toll-like Receptor 4 Ligand That Induces Human Dendritic Cell Secretion of IL-1β. PLoS ONE. 2017;12:e0176793. doi: 10.1371/journal.pone.0176793. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

