3K3A-Activated Protein C Inhibits Choroidal Neovascularization Growth and Leakage and Reduces NLRP3 Inflammasome, IL-1β, and Inflammatory Cell Accumulation in the Retina
- PMID: 37445820
- PMCID: PMC10341424
- DOI: 10.3390/ijms241310642
3K3A-Activated Protein C Inhibits Choroidal Neovascularization Growth and Leakage and Reduces NLRP3 Inflammasome, IL-1β, and Inflammatory Cell Accumulation in the Retina
Abstract
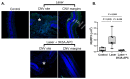
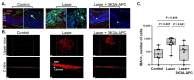
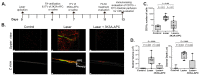
3K3A-Activated Protein C (APC) is a recombinant variant of the physiological anticoagulant APC with cytoprotective properties and reduced bleeding risks. We studied the potential use of 3K3A-APC as a multi-target therapeutic option for choroidal neovascularization (CNV), a common cause of vision loss in age-related macular degeneration. CNV was induced by laser photocoagulation in a murine model, and 3K3A-APC was intravitreally injected. The impact of 3K3A-APC treatment on myeloid and microglia cell activation and recruitment and on NLRP3 inflammasome, IL-1β, and VEGF levels was assessed using cryosection, retinal flat-mount immunohistochemistry and vascular imaging. Additionally, we evaluated the use of fluorescein angiography as a surrogate marker for in vivo evaluation of the efficacy of 3K3A-APC treatment against leaking CNV lesions. Our results demonstrated that 3K3A-APC treatment significantly reduced the accumulation and activation of myeloid cells and microglia in the CNV area and decreased the NLRP3 and IL-1β levels at the CNV site and the surrounding retina. Furthermore, 3K3A-APC treatment resulted in leakage regression and CNV growth suppression. These findings indicate that the anti-inflammatory activities of 3K3A-APC contribute to CNV inhibition. Our study suggests the potential use of 3K3A-APC as a novel multi-target treatment for CNV.
Keywords: activated protein C; choroidal neovascularization; inflammation NLRP3; microglia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Activated Protein C (APC) and 3K3A-APC-Induced Regression of Choroidal Neovascularization (CNV) Is Accompanied by Vascular Endothelial Growth Factor (VEGF) Reduction.Biomolecules. 2021 Feb 26;11(3):358. doi: 10.3390/biom11030358. Biomolecules. 2021. PMID: 33652861 Free PMC article.
-
3K3A-Activated Protein C Prevents Microglia Activation, Inhibits NLRP3 Inflammasome and Limits Ocular Inflammation.Int J Mol Sci. 2022 Nov 17;23(22):14196. doi: 10.3390/ijms232214196. Int J Mol Sci. 2022. PMID: 36430674 Free PMC article.
-
Activated protein C induces suppression and regression of choroidal neovascularization- A murine model.Exp Eye Res. 2019 Sep;186:107695. doi: 10.1016/j.exer.2019.107695. Epub 2019 Jun 12. Exp Eye Res. 2019. PMID: 31201804
-
Distinct effects of complement and of NLRP3- and non-NLRP3 inflammasomes for choroidal neovascularization.Elife. 2020 Dec 11;9:e60194. doi: 10.7554/eLife.60194. Elife. 2020. PMID: 33305736 Free PMC article.
-
Role of inflammasome activation in neovascular age-related macular degeneration.FEBS J. 2023 Jan;290(1):28-36. doi: 10.1111/febs.16278. Epub 2021 Dec 4. FEBS J. 2023. PMID: 34767301 Free PMC article. Review.
Cited by
-
NLRP3 and autophagy in retinal ganglion cell inflammation in age-related macular degeneration: potential therapeutic implications.Int J Ophthalmol. 2024 Aug 18;17(8):1531-1544. doi: 10.18240/ijo.2024.08.20. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39156786 Free PMC article. Review.
-
Revamping anti-cGAS-STING therapy via an injectable thermo-responsive supramolecular hydrogel for pathological retinal angiogenesis.Asian J Pharm Sci. 2024 Oct;19(5):100969. doi: 10.1016/j.ajps.2024.100969. Epub 2024 Sep 21. Asian J Pharm Sci. 2024. PMID: 39474128 Free PMC article.
-
Platelets at the intersection of inflammation and coagulation in the APC-mediated response to myocardial ischemia/reperfusion injury.FASEB J. 2024 Aug 31;38(16):e23890. doi: 10.1096/fj.202401128R. FASEB J. 2024. PMID: 39143722 Review.
-
An orthosteric/allosteric bivalent peptide agonist comprising covalently linked protease-activated receptor-derived peptides mimics in vitro and in vivo activities of activated protein C.J Thromb Haemost. 2024 Jul;22(7):2039-2051. doi: 10.1016/j.jtha.2024.04.007. Epub 2024 Apr 24. J Thromb Haemost. 2024. PMID: 38670314 Free PMC article.
References
-
- Wong J.H.C., Ma J.Y.W., Jobling A.I., Brandli A., Greferath U., Fletcher E.L., Vessey K.A. Exploring the Pathogenesis of Age-Related Macular Degeneration: A Review of the Interplay between Retinal Pigment Epithelium Dysfunction and the Innate Immune System. Front. Neurosci. 2022;16:1871. doi: 10.3389/fnins.2022.1009599. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

