Unlocking the Potential of Arginine Deprivation Therapy: Recent Breakthroughs and Promising Future for Cancer Treatment
- PMID: 37445845
- PMCID: PMC10341449
- DOI: 10.3390/ijms241310668
Unlocking the Potential of Arginine Deprivation Therapy: Recent Breakthroughs and Promising Future for Cancer Treatment
Abstract
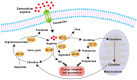
Arginine is a semi-essential amino acid that supports protein synthesis to maintain cellular functions. Recent studies suggest that arginine also promotes wound healing, cell division, ammonia metabolism, immune system regulation, and hormone biosynthesis-all of which are critical for tumor growth. These discoveries, coupled with the understanding of cancer cell metabolic reprogramming, have led to renewed interest in arginine deprivation as a new anticancer therapy. Several arginine deprivation strategies have been developed and entered clinical trials. The main principle behind these therapies is that arginine auxotrophic tumors rely on external arginine sources for growth because they carry reduced key arginine-synthesizing enzymes such as argininosuccinate synthase 1 (ASS1) in the intracellular arginine cycle. To obtain anticancer effects, modified arginine-degrading enzymes, such as PEGylated recombinant human arginase 1 (rhArg1-PEG) and arginine deiminase (ADI-PEG 20), have been developed and shown to be safe and effective in clinical trials. They have been tried as a monotherapy or in combination with other existing therapies. This review discusses recent advances in arginine deprivation therapy, including the molecular basis of extracellular arginine degradation leading to tumor cell death, and how this approach could be a valuable addition to the current anticancer arsenal.
Keywords: ADI-PEG 20; BCT-100; arginine auxotrophic cancer; arginine deprivation cancer therapy; biomarker; circulating arginine; rhArg1-PEG.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

