Combining Solid and Liquid Biopsy for Therapy Monitoring in Esophageal Cancer
- PMID: 37445849
- PMCID: PMC10341643
- DOI: 10.3390/ijms241310673
Combining Solid and Liquid Biopsy for Therapy Monitoring in Esophageal Cancer
Abstract
Esophageal cancer (EC) has one of the highest mortality rates among cancers, making it imperative that therapies are optimized and dynamically adapted to individuals. In this regard, liquid biopsy is an increasingly important method for residual disease monitoring. However, conflicting detection rates (14% versus 60%) and varying cell-free circulating tumor DNA (ctDNA) levels (0.07% versus 0.5%) have been observed in previous studies. Here, we aim to resolve this discrepancy. For 19 EC patients, a complete set of cell-free DNA (cfDNA), formalin-fixed paraffin-embedded tumor tissue (TT) DNA and leukocyte DNA was sequenced (139 libraries). cfDNA was examined in biological duplicates and/or longitudinally, and TT DNA was examined in technical duplicates. In baseline cfDNA, mutations were detected in 12 out of 19 patients (63%); the median ctDNA level was 0.4%. Longitudinal ctDNA changes were consistent with clinical presentation. Considerable mutational diversity was observed in TT, with fewer mutations in cfDNA. The most recurrently mutated genes in TT were TP53, SMAD4, TSHZ3, and SETBP1, with SETBP1 being reported for the first time. ctDNA in blood can be used for therapy monitoring of EC patients. However, a combination of solid and liquid samples should be used to help guide individualized EC therapy.
Keywords: EC; NGS; SETBP1; cell-free DNA; cfDNA; circulating tumor DNA; ctDNA; sequencing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
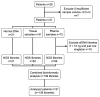
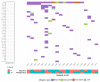
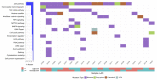
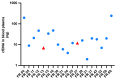
Similar articles
-
Detection of Circulating Tumor DNA in Plasma: A Potential Biomarker for Esophageal Adenocarcinoma.Ann Thorac Surg. 2019 Aug;108(2):343-349. doi: 10.1016/j.athoracsur.2019.04.004. Epub 2019 May 3. Ann Thorac Surg. 2019. PMID: 31059681 Free PMC article.
-
Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma.Cancer Sci. 2019 Feb;110(2):617-628. doi: 10.1111/cas.13906. Epub 2019 Jan 25. Cancer Sci. 2019. PMID: 30536551 Free PMC article.
-
Identification of liquid biopsy-based mutations in colorectal cancer by targeted sequencing assays.Mol Cell Probes. 2023 Feb;67:101888. doi: 10.1016/j.mcp.2022.101888. Epub 2022 Dec 10. Mol Cell Probes. 2023. PMID: 36513244
-
Tumor-specific genetic aberrations in cell-free DNA of gastroesophageal cancer patients.J Gastroenterol. 2019 Feb;54(2):108-121. doi: 10.1007/s00535-018-1508-5. Epub 2018 Sep 21. J Gastroenterol. 2019. PMID: 30242476 Review.
-
A Review of Circulating Tumor DNA in the Diagnosis and Monitoring of Esophageal Cancer.Med Sci Monit. 2022 Feb 25;28:e934106. doi: 10.12659/MSM.934106. Med Sci Monit. 2022. PMID: 35210388 Free PMC article. Review.
Cited by
-
ctDNA responds to neoadjuvant treatment in locally advanced rectal cancer.J Cancer Res Clin Oncol. 2024 Sep 22;150(9):428. doi: 10.1007/s00432-024-05944-7. J Cancer Res Clin Oncol. 2024. PMID: 39307893 Free PMC article.
References
-
- Van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., Richel D.J., Nieuwenhuijzen G.A., Hospers G.A., Bonenkamp J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. - DOI - PubMed
-
- Hsu H.Y., Chao Y.K., Hsieh C.H., Wen Y.W., Chang H.K., Tseng C.K., Liu Y.H. Postoperative Adjuvant Therapy Improves Survival in Pathologic Nonresponders After Neoadjuvant Chemoradiation for Esophageal Squamous Cell Carcinoma: A Propensity-Matched Analysis. Ann. Thorac. Surg. 2016;102:1687–1693. doi: 10.1016/j.athoracsur.2016.05.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

