Neural Regeneration in Dry Eye Secondary to Systemic Lupus Erythematosus Is Also Disrupted like in Rheumatoid Arthritis, but in a Progressive Fashion
- PMID: 37445856
- PMCID: PMC10341867
- DOI: 10.3390/ijms241310680
Neural Regeneration in Dry Eye Secondary to Systemic Lupus Erythematosus Is Also Disrupted like in Rheumatoid Arthritis, but in a Progressive Fashion
Abstract
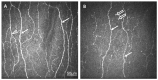
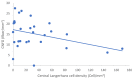
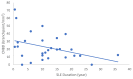
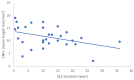
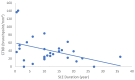
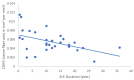
Our objective in this study was to analyze the aberrant neural regeneration activity in the cornea by means of in vivo confocal microscopy in systemic lupus erythematosus patients with concurrent dry eye disease. We examined 29 systemic lupus erythematosus patients and 29 age-matched healthy control subjects. Corneal nerve fiber density (CNFD, the number of fibers/mm2) and peripheral Langerhans cell morphology were lower (p < 0.05) in systemic lupus erythematosus patients compared to the control group. Interestingly, corneal nerve branch density, corneal nerve fiber length, corneal nerve fiber total branch density, and corneal nerve fiber area showed a negative correlation with disease duration. A negative correlation was also demonstrated between average corneal nerve fiber density and central Langerhans cell density. This is in line with our hypothesis that corneal somatosensory terminal Piezo2 channelopathy-induced impaired Piezo2-Piezo1 crosstalk not only disrupts regeneration and keeps transcription activated, but could lead to Piezo1 downregulation and cell activation on Langerhans cells when we consider a chronic path. Hence, Piezo2 containing mechanosensory corneal nerves and dendritic Langerhans cells could also be regarded as central players in shaping the ocular surface neuroimmune homeostasis through the Piezo system. Moreover, lost autoimmune neuroinflammation compensation, lost phagocytic self-eating capacity, and lost transcription regulation, not to mention autoantibodies against vascular heparin sulfate proteoglycans and phospholipids, could all contribute to the progressive fashion of dry eye disease in systemic lupus erythematosus.
Keywords: Langerhans cell; Piezo1; Piezo2; autoimmune disease; channelopathy; dry eye disease; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Disrupted Neural Regeneration in Dry Eye Secondary to Ankylosing Spondylitis-With a Theoretical Link between Piezo2 Channelopathy and Gateway Reflex, WDR Neurons, and Flare-Ups.Int J Mol Sci. 2023 Oct 22;24(20):15455. doi: 10.3390/ijms242015455. Int J Mol Sci. 2023. PMID: 37895134 Free PMC article.
-
Evidence of Disruption in Neural Regeneration in Dry Eye Secondary to Rheumatoid Arthritis.Int J Mol Sci. 2023 Apr 19;24(8):7514. doi: 10.3390/ijms24087514. Int J Mol Sci. 2023. PMID: 37108693 Free PMC article.
-
Subclinical Corneal Nerve Fiber Damage and Immune Cell Activation in Systemic Lupus Erythematosus: A Corneal Confocal Microscopy Study.Transl Vis Sci Technol. 2021 Dec 1;10(14):10. doi: 10.1167/tvst.10.14.10. Transl Vis Sci Technol. 2021. PMID: 34905000 Free PMC article.
-
Is the Sex Difference a Clue to the Pathomechanism of Dry Eye Disease? Watch out for the NGF-TrkA-Piezo2 Signaling Axis and the Piezo2 Channelopathy.J Mol Neurosci. 2022 Aug;72(8):1598-1608. doi: 10.1007/s12031-022-02015-9. Epub 2022 May 4. J Mol Neurosci. 2022. PMID: 35507012 Free PMC article. Review.
-
Application of In Vivo Confocal Microscopy in Dry Eye Disease.Invest Ophthalmol Vis Sci. 2018 Nov 1;59(14):DES41-DES47. doi: 10.1167/iovs.17-23602. Invest Ophthalmol Vis Sci. 2018. PMID: 30481805 Review.
Cited by
-
Unveiling Ocular Manifestations in Systemic Lupus Erythematosus.J Clin Med. 2024 Feb 12;13(4):1047. doi: 10.3390/jcm13041047. J Clin Med. 2024. PMID: 38398361 Free PMC article. Review.
-
Squishy matters - Corneal mechanobiology in health and disease.Prog Retin Eye Res. 2024 Mar;99:101234. doi: 10.1016/j.preteyeres.2023.101234. Epub 2024 Jan 2. Prog Retin Eye Res. 2024. PMID: 38176611 Free PMC article. Review.
-
Disrupted Neural Regeneration in Dry Eye Secondary to Ankylosing Spondylitis-With a Theoretical Link between Piezo2 Channelopathy and Gateway Reflex, WDR Neurons, and Flare-Ups.Int J Mol Sci. 2023 Oct 22;24(20):15455. doi: 10.3390/ijms242015455. Int J Mol Sci. 2023. PMID: 37895134 Free PMC article.
References
-
- El-Akhras B.A., Talaat R.M., El-Masry S.A., Bassyouni I.H., El-Sayed I.H., Ali Y.B. Crosstalk between miR-146a and pro-inflammatory cytokines in patients with systemic lupus erythematosus. Int. J. Immunopathol. Pharmacol. 2023;37:3946320231154998. doi: 10.1177/03946320231154998. - DOI - PMC - PubMed
-
- Nicolle P., Liang H., Reboussin E., Rabut G., Warcoin E., Brignole-Baudouin F., Melik-Parsadaniantz S., Baudouin C., Labbe A., Reaux-Le Goazigo A. Proinflammatory Markers, Chemokines, and Enkephalin in Patients Suffering from Dry Eye Disease. Int. J. Mol. Sci. 2018;19:1221. doi: 10.3390/ijms19041221. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

