Research Advances on the Bioactivity of 1,2,3-Triazolium Salts
- PMID: 37445872
- PMCID: PMC10341799
- DOI: 10.3390/ijms241310694
Research Advances on the Bioactivity of 1,2,3-Triazolium Salts
Abstract
1,2,3-Triazolium salts have demonstrated significant potential in the fields of medicine and agriculture, exhibiting exceptional antibacterial, antifungal, anticancer, and antileishmanial properties. Moreover, these salts can be utilized as additives or components to produce nano- and fiber-based materials with antibacterial properties. In this review, we summarize several synthetic strategies to obtain 1,2,3-triazolium salts and the structures of 1,2,3-triazolium derivatives with biological activities in the domains of pharmaceuticals, pesticides, and functional materials. Additionally, the structure-activity relationship (SAR) of 1,2,3-triazolium salts with different biological activities has been analyzed. Finally, this review presents the potential applications and prospects of 1,2,3-triazolium salts in the fields of agriculture, medicine, and industrial synthesis.
Keywords: 1,2,3-triazolium salts; SAR; antibacterial; anticancer; antifungal; antileishmanial; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
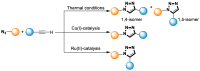
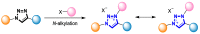
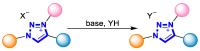
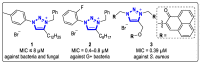
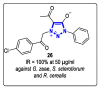


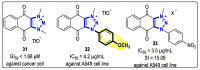
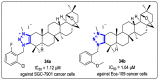
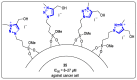




References
-
- Xie M., Tang H., Zhang Y., Guo Z., Guo H., Qu G. A new method for the synthesis of alkyl-substituted 1,2,3-triazole compounds. Chin. J. Org. Chem. 2015;35:2589–2594. doi: 10.6023/cjoc201505040. - DOI
-
- Aizpurua J.M., Fratila R.M., Monasterio Z., Pérez-Esnaola N., Andreieff E., Irastorzaa A., Sagartzazu-Aizpuruaa M. Triazolium cations: From the “click” pool to multipurpose applications. New J. Chem. 2014;38:474–480. doi: 10.1039/C3NJ00667K. - DOI
-
- Yacob Z., Liebscher J. 1,2,3-Triazolium salts as a versatile new class of ionic liquids. In: Handy S.T., editor. Ionic Liquids. Volume 1. IntechOpen; Rijeka, Germany: 2011. pp. 3–23. - DOI
-
- Li Q., Qiu L., Tan W., Gu G., Guo Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017;7:42225–42232. doi: 10.1039/C7RA08244D. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 111 Program, D20023/Program of Introducing Talents of Discipline to Universities of China at Guizhou University
- Qiankehe Achievement [2022] General 063/The Science and Technology Plan Project (Topic), Guizhou Province
- Qianjiaohe KY number (2020)004/Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

