Skin Wound following Irradiation Aggravates Radiation-Induced Brain Injury in a Mouse Model
- PMID: 37445879
- PMCID: PMC10341425
- DOI: 10.3390/ijms241310701
Skin Wound following Irradiation Aggravates Radiation-Induced Brain Injury in a Mouse Model
Abstract
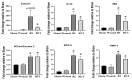
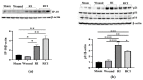
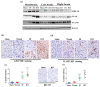
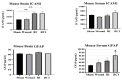
Radiation injury- and radiation combined with skin injury-induced inflammatory responses in the mouse brain were evaluated in this study. Female B6D2F1/J mice were subjected to a sham, a skin wound (SW), 9.5 Gy 60Co total-body gamma irradiation (RI), or 9.5 Gy RI combined with a skin puncture wound (RCI). Survival, body weight, and wound healing were tracked for 30 days, and mouse brain samples were collected on day 30 after SW, RI, RCI, and the sham control. Our results showed that RCI caused more severe animal death and body weight loss compared with RI, and skin wound healing was significantly delayed by RCI compared to SW. RCI and RI increased the chemokines Eotaxin, IP-10, MIG, 6Ckine/Exodus2, MCP-5, and TIMP-1 in the brain compared to SW and the sham control mice, and the Western blot results showed that IP-10 and p21 were significantly upregulated in brain cells post-RI or -RCI. RI and RCI activated both astrocytes and endothelial cells in the mouse brain, subsequently inducing blood-brain barrier (BBB) leakage, as shown by the increased ICAM1 and GFAP proteins in the brain and GFAP in the serum. The Doublecortin (DCX) protein, the "gold standard" for measuring neurogenesis, was significantly downregulated by RI and RCI compared with the sham group. Furthermore, RI and RCI decreased the expression of the neural stem cell marker E-cadherin, the intermediate progenitor marker MASH1, the immature neuron cell marker NeuroD1, and the mature neuron cell marker NeuN, indicating neural cell damage in all development stages after RI and RCI. Immunohistochemistry (IHC) staining further confirmed the significant loss of neural cells in RCI. Our data demonstrated that RI and RCI induced brain injury through inflammatory pathways, and RCI exacerbated neural cell damage more than RI.
Keywords: blood–brain barrier leakage; brain injury; inflammation; neural cell damage; radiation; radiation combined injury; skin wound.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- DiCarlo A.L., Hatchett R.J., Kaminski J.M., Ledney G.D., Pellmar T.C., Okunieff P., Ramakrishnan N. Medical Countermeasures for Radiation Combined Injury: Radiation with Burn, Blast, Trauma and/or Sepsis. Report of an NIAID Workshop, March 26–27, 2007. Radiat. Res. 2008;169:712–721. doi: 10.1667/RR1295.1. - DOI - PMC - PubMed
-
- Wang L., Zhai M., Lin B., Cui W., Hull L., Li X., Anderson M.N., Smith J.T., Umali M.V., Jiang S., et al. PEG-G-CSF and L-Citrulline Combination Therapy for Mitigating Skin Wound Combined Radiation Injury in a Mouse Model. Radiat. Res. 2021;196:113–127. doi: 10.1667/RADE-20-00151.1. - DOI - PMC - PubMed
-
- Hauer-Jensen M., Kumar K.S., Wang J., Berbee M., Fu Q., Boerma M. Intestinal Toxicity in Radiation- and Combined Injury: Significance, Mechanisms, and Countermeasures: 2008. Nova Science Publishers, Inc.; New York, NY, USA: 2008. pp. 61–100.
-
- Kiang J.G., Jiao W., Cary L.H., Mog S.R., Elliott T.B., Pellmar T.C., Ledney G.D. Wound Trauma Increases Radiation-Induced Mortality by Activation of iNOS Pathway and Elevation of Cytokine Concentrations and Bacterial Infection. Radiat. Res. 2010;173:319–332. doi: 10.1667/RR1892.1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

