Cardioprotective Effects of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension Are Mediated by the Local Reduction of Sympathetic Activity and Inflammation
- PMID: 37445888
- PMCID: PMC10341774
- DOI: 10.3390/ijms241310710
Cardioprotective Effects of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension Are Mediated by the Local Reduction of Sympathetic Activity and Inflammation
Abstract
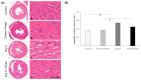
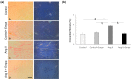
The cardioprotective effects of sodium glucose cotrasponter 2 (SGLT2) inhibitors seem to be independent from the effects on glycemic control, through little-known mechanisms. In this study, we investigate whether the cardioprotective effects of empagliflozin, a SGLT2 inhibitor, may be associated with myocardial sympathetic activity and inflammatory cell infiltration in an experimental model of angiotensin II-dependent hypertension. Angiotensin II (Ang II), Ang II plus Empagliflozin, physiological saline, or physiological saline plus empagliflozin were administered to Sprague Dawley rats for two weeks. Blood pressure was measured by plethysmographic method. Myocardial hypertrophy and fibrosis were analysed by histomorphometry, and inflammatory cell infiltration and tyrosine hydroxylase expression, implemented as a marker of sympathetic activity, were evaluated by immunohistochemistry. Ang II increased blood pressure, myocardial hypertrophy, fibrosis, inflammatory infiltrates and tyrosine hydroxylase expression, as compared to the control group. Empagliflozin administration prevented the development of myocardial hypertrophy, fibrosis, inflammatory infiltrates and tyrosine hydroxylase overexpression in Ang II-treated rats, without affecting blood glucose and the Ang II-dependent increase in blood pressure. These data demonstrate that the cardioprotective effects of SGLT2 inhibition in Ang II-dependent hypertension may result from the myocardial reduction of sympathetic activity and inflammation and are independent of the modulation of blood pressure and blood glucose levels.
Keywords: SGLT2-inhibitors; angiotensin II; hypertension; inflammatory infiltrates; myocardial fibrosis; myocardial hypertrophy; rats; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Angiotensin Type 2 and Mas Receptor Activation Prevents Myocardial Fibrosis and Hypertrophy through the Reduction of Inflammatory Cell Infiltration and Local Sympathetic Activity in Angiotensin II-Dependent Hypertension.Int J Mol Sci. 2021 Dec 20;22(24):13678. doi: 10.3390/ijms222413678. Int J Mol Sci. 2021. PMID: 34948475 Free PMC article.
-
Renal Anti-Fibrotic Effect of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension.Am J Nephrol. 2020;51(2):119-129. doi: 10.1159/000505144. Epub 2020 Jan 7. Am J Nephrol. 2020. PMID: 31910407
-
Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin II-dependent kidney damage.BMC Nephrol. 2019 Aug 2;20(1):292. doi: 10.1186/s12882-019-1490-z. BMC Nephrol. 2019. PMID: 31375080 Free PMC article.
-
Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure.Vasc Health Risk Manag. 2016 Oct 27;12:393-405. doi: 10.2147/VHRM.S111991. eCollection 2016. Vasc Health Risk Manag. 2016. PMID: 27822054 Free PMC article. Review.
-
An evaluation of empagliflozin and it's applicability to hypertension as a therapeutic option.Expert Opin Pharmacother. 2020 Jul;21(10):1157-1166. doi: 10.1080/14656566.2020.1751815. Epub 2020 Apr 17. Expert Opin Pharmacother. 2020. PMID: 32301361 Review.
Cited by
-
The Off-Target Cardioprotective Mechanisms of Sodium-Glucose Cotransporter 2 Inhibitors: An Overview.Int J Mol Sci. 2024 Jul 14;25(14):7711. doi: 10.3390/ijms25147711. Int J Mol Sci. 2024. PMID: 39062954 Free PMC article. Review.
-
Empagliflozin Dampens Doxorubicin-Induced Chemobrain in Rats: The Possible Involvement of Oxidative Stress and PI3K/Akt/mTOR/NF-κB/TNF-α Signaling Pathways.Mol Neurobiol. 2025 Mar;62(3):3480-3492. doi: 10.1007/s12035-024-04499-5. Epub 2024 Sep 20. Mol Neurobiol. 2025. PMID: 39302617
-
Measurement of blood pressure in rats: Invasive or noninvasive methods?Physiol Rep. 2024 Sep;12(17):e70041. doi: 10.14814/phy2.70041. Physiol Rep. 2024. PMID: 39266877 Free PMC article. Review.
-
Sodium Glucose Cotransporter-2 Inhibitors in Non-Diabetic Kidney Disease: Evidence in Experimental Models.Pharmaceuticals (Basel). 2024 Mar 11;17(3):362. doi: 10.3390/ph17030362. Pharmaceuticals (Basel). 2024. PMID: 38543148 Free PMC article. Review.
-
Neuroimmune Interactions and Their Role in Immune Cell Trafficking in Cardiovascular Diseases and Cancer.Int J Mol Sci. 2025 Mar 12;26(6):2553. doi: 10.3390/ijms26062553. Int J Mol Sci. 2025. PMID: 40141195 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

