Persistence of Anti-S1 IgG against SARS-CoV-2 Eight Months after the Booster Dose of Vaccine in Naive and Previously Infected Healthcare Workers
- PMID: 37445893
- PMCID: PMC10341794
- DOI: 10.3390/ijms241310713
Persistence of Anti-S1 IgG against SARS-CoV-2 Eight Months after the Booster Dose of Vaccine in Naive and Previously Infected Healthcare Workers
Abstract
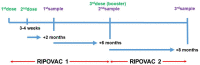
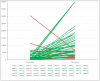
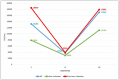
Our aim was to evaluate the immune response of healthcare workers included in the RIPOVAC study, after receiving a booster dose (third dose), in terms of intensity and persistence of induced antibodies. In the second phase of the RIPOVAC study, between December 2021 and January 2022, eight months after the second dose, 389 voluntary, immunocompetent, non-pregnant healthcare workers received a booster dose of SARS-CoV-2 vaccine, and a serum sample was obtained. Two groups of patients were established: with and without previous SARS-CoV-2 infection. In order to quantify anti-S1 IgG (AU/mL) we used CMIA (Abbott). All of the health workers were anti-S IgG positive 8 months after receiving the booster dose of the vaccine, with a mean of 17,040 AU/mL. In 53 patients without previous infection, antibody levels increased by a mean of 10,762 AU/mL. This figure is seven times higher than the one produced after the second dose (1506 AU/mL). The booster dose produces a robust elevation of the antibody level, which persists at 8 months, with levels significantly higher than those reached after the second dose, which allow one to predict a persistence of more than one year. The study demonstrates the efficacy of the booster dose of anti-SARS-CoV-2 vaccines.
Keywords: SARS-CoV-2; booster vaccine; post-vaccine IgG antibody persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Serrano L., Algarate S., Herrero-Cortina B., Bueno J., González-Barriga M.T., Ducons M., Montero-Marco J., Acha B., Taboada A., Sanz-Burillo P., et al. Assessment of humoral immune response to two mRNA SARS-CoV-2 vaccines (Moderna and Pfizer) in healthcare workers fully vaccinated with and without a history of previous infection. J. Appl. Microbiol. 2022;133:1969–1974. doi: 10.1111/jam.15699. - DOI - PMC - PubMed
-
- Núñez López C., González de Abreu J.M., Pérez-Blanco V., de Miguel Buckley R., Romero Gómez M.P., Díaz-Menéndez M. La Paz Health Care Workers Vaccination Study Group; Appendix. La Paz Health Care Workers Vaccination Study Group. Effectiveness of the BNT162b2 mRNA Covid-19 vaccine in Spanish healthcare workers. Enferm. Infecc. Microbiol. Clin. 2023;41:33–35. doi: 10.1016/j.eimc.2021.06.021. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

