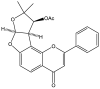
Ameliorative Potential of (-) Pseudosemiglabrin in Mice with Pilocarpine-Induced Epilepsy: Antioxidant, Anti-Inflammatory, Anti-Apoptotic, and Neurotransmission Modulation
- PMID: 37445950
- PMCID: PMC10341902
- DOI: 10.3390/ijms241310773
Ameliorative Potential of (-) Pseudosemiglabrin in Mice with Pilocarpine-Induced Epilepsy: Antioxidant, Anti-Inflammatory, Anti-Apoptotic, and Neurotransmission Modulation
Abstract
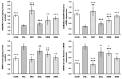
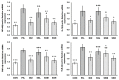
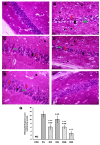
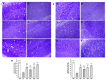
One prevalent neurological disorder is epilepsy. Modulating GABAergic/glutamatergic neurotransmission, Nrf2/HO-1, PI3K/Akt, and TLR-4/NF-B pathways might be a therapeutic strategy for epilepsy. Eight-week-old BALB/c mice were administered 12.5, 25, or 50 mg/kg (-) pseudosemiglabrin orally one hour before inducing epilepsy with an i.p. injection of 360 mg/kg pilocarpine. (-) Pseudosemiglabrin dose-dependently alleviated pilocarpine-induced epilepsy, as revealed by the complete repression of pilocarpine-induced convulsions and 100% survival rate in mice. Furthermore, (-) pseudosemiglabrin significantly enhanced mice's locomotor activities, brain GABA, SLC1A2, GABARα1 levels, glutamate decarboxylase activity, and SLC1A2 and GABARα1mRNA expression while decreasing brain glutamate, SLC6A1, GRIN1 levels, GABA transaminase activity, and SLC6A1 and GRIN1 mRNA expression. These potentials can be due to the suppression of the TLR-4/NF-κB and the enhancement of the Nrf2/HO-1 and PI3K/Akt pathways, as demonstrated by the reduction in TLR-4, NF-κB, IL-1β, TNF-α mRNA expression, MDA, NO, caspase-3, Bax levels, and Bax/Bcl-2 ratio, and the enhancement of Nrf2, HO-1, PI3K, Akt mRNA expression, GSH, Bcl-2 levels, and SOD activity. Additionally, (-) pseudosemiglabrin abrogated the pilocarpine-induced histopathological changes. Interestingly, the (-) pseudosemiglabrin intervention showed a comparable effect to the standard medication, diazepam. Therefore, (-) pseudosemiglabrin can be a promising medication for the management of epilepsy.
Keywords: (-) pseudosemiglabrin; GABAergic and glutamatergic neurotransmission; Nrf2/HO-1 pathway; PI3K/Akt pathway; TLR-4/NF-κB pathway; epilepsy.
Conflict of interest statement
The authors declare no conflict of interest. Moreover, the funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- WHO New WHO Brief Sets out Actions Needed to Improve Lives of People with Epilepsy. [(accessed on 5 January 2023)]; Available online: https://www.who.int/news/item/12-12-2022-new-who-brief-sets-out-actions-....
-
- Simonato M., Löscher W., Cole A.J., Dudek F.E., Engel J., Jr., Kaminski R.M., Loeb J.A., Scharfman H., Staley K.J., Velíšek L. Finding a better drug for epilepsy: Preclinical screening strategies and experimental trial design. Epilepsia. 2012;53:1860–1867. doi: 10.1111/j.1528-1167.2012.03541.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

