Homocysteine and Glaucoma
- PMID: 37445966
- PMCID: PMC10341544
- DOI: 10.3390/ijms241310790
Homocysteine and Glaucoma
Abstract
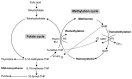
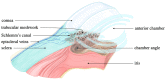
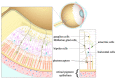
Elevated levels of homocysteine (Hcy), a non-proteinogenic amino acid, may lead to a host of manifestations across the biological systems, particularly the nervous system. Defects in Hcy metabolism have been associated with many neurodegenerative diseases including glaucoma, i.e., the leading cause of blindness. However, the pathophysiology of elevated Hcy and its eligibility as a risk factor for glaucoma remain unclear. We aimed to provide a comprehensive review of the relationship between elevated Hcy levels and glaucoma. Through a systemic search of the PubMed and Google Scholar databases, we found that elevated Hcy might play an important role in the pathogenesis of glaucoma. Further research will be necessary to help clarify the specific contribution of elevated Hcy in the pathogenesis of glaucoma. A discovery and conceptual understanding of Hcy-associated glaucoma could be the keys to providing better therapeutic treatment, if not prophylactic treatment, for this disease.
Keywords: aqueous humor; exfoliation glaucoma; glaucoma; homocysteine; primary open-angle glaucoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Butz L.W., du Vigneaud V. The formation of a homologue of cystine by the decomposition of methionine with sulfuric acid. J. Biol. Chem. 1932;99:135–142. doi: 10.1016/S0021-9258(18)76074-2. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

