Protective Effect of Amaranthus cruentus L. Seed Oil on UVA-Radiation-Induced Apoptosis in Human Skin Fibroblasts
- PMID: 37445970
- PMCID: PMC10341579
- DOI: 10.3390/ijms241310795
Protective Effect of Amaranthus cruentus L. Seed Oil on UVA-Radiation-Induced Apoptosis in Human Skin Fibroblasts
Abstract
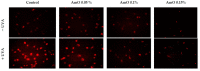
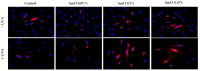
Since the exposure of fibroblasts to prolonged UVA radiation induces oxidative stress and apoptosis, there is a need for effective skin protection compounds with cytoprotective and antioxidant properties. One of their sources is Amaranthus cruentus L. seed oil (AmO), which is rich in unsaturated fatty acids, squalene, vitamin E derivatives and phytosterols. The aim of this study was to evaluate whether AmO evokes a protective effect on the apoptosis induced by UVA radiation in human skin fibroblasts. UVA radiation at an applied dose of 10 J/cm2 caused a significant reduction in the survival of human skin fibroblasts and directed them into the apoptosis pathway. Increased expression of p53, caspase-3, caspase-9 and PARP proteins in UVA-treated fibroblasts suggests the intrinsic mechanism of apoptosis. Application of the oil at 0.1% and 0.15% concentrations to UVA-treated cells decreased the expression of these proteins, which was accompanied by increased cell survival. Similarly, the UVA-dependent decrease in the expression of p-Akt and mTOR proteins was restored under the effect of the studied oil. The molecular mechanism of this phenomenon was related to the stimulation of antioxidant processes through the activation of Nrf2. This suggests that AmO stimulated the antioxidant system in fibroblasts, preventing the effects of UVA-induced oxidative stress, which may lead to pharmaceutical and cosmetological applications as a sun-protective substance.
Keywords: Amaranthus cruentus L. seed oil; UVA radiation; antioxidant; apoptosis; cosmetology; dermal fibroblasts; oxidative stress; pharmacy; sun-protective substance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Amaranthus cruentus L. Seed Oil Counteracts UVA-Radiation-Induced Inhibition of Collagen Biosynthesis and Wound Healing in Human Skin Fibroblasts.Int J Mol Sci. 2024 Jan 11;25(2):925. doi: 10.3390/ijms25020925. Int J Mol Sci. 2024. PMID: 38256000 Free PMC article.
-
Caffeic acid derivatives isolated from Galinsoga parviflora herb protected human dermal fibroblasts from UVA-radiation.Phytomedicine. 2019 Apr;57:215-222. doi: 10.1016/j.phymed.2018.12.022. Epub 2018 Dec 17. Phytomedicine. 2019. PMID: 30785017
-
Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation.Arch Dermatol Res. 2019 Apr;311(3):203-219. doi: 10.1007/s00403-019-01898-w. Epub 2019 Feb 19. Arch Dermatol Res. 2019. PMID: 30783768
-
Ultraviolet A protective potential of plant extracts and phytochemicals.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Mar;164(1):1-22. doi: 10.5507/bp.2020.010. Epub 2020 Mar 17. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020. PMID: 32188958 Review.
-
Effect of ultraviolet radiation on the Nrf2 signaling pathway in skin cells.Int J Radiat Biol. 2021;97(10):1383-1403. doi: 10.1080/09553002.2021.1962566. Epub 2021 Aug 19. Int J Radiat Biol. 2021. PMID: 34338112 Review.
Cited by
-
The Mechanism of Protective Action of Plant-Derived Squalane (2,6,10,15,19,23-Hexamethyltetracosane) Against UVA Radiation-Induced Apoptosis in Human Dermal Fibroblasts.Antioxidants (Basel). 2025 Jul 11;14(7):853. doi: 10.3390/antiox14070853. Antioxidants (Basel). 2025. PMID: 40722956 Free PMC article.
-
Squalane as a Promising Agent Protecting UV-Induced Inhibition of Collagen Biosynthesis and Wound Healing in Human Dermal Fibroblast.Molecules. 2025 Apr 29;30(9):1964. doi: 10.3390/molecules30091964. Molecules. 2025. PMID: 40363772 Free PMC article.
-
Amaranthus cruentus L. Seed Oil Counteracts UVA-Radiation-Induced Inhibition of Collagen Biosynthesis and Wound Healing in Human Skin Fibroblasts.Int J Mol Sci. 2024 Jan 11;25(2):925. doi: 10.3390/ijms25020925. Int J Mol Sci. 2024. PMID: 38256000 Free PMC article.
-
Natural Modulators of Key Signaling Pathways in Skin Inflammageing.Clin Cosmet Investig Dermatol. 2024 Dec 18;17:2967-2988. doi: 10.2147/CCID.S502252. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39712942 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

