The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole
- PMID: 37445975
- PMCID: PMC10342039
- DOI: 10.3390/ijms241310793
The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole
Abstract
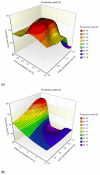
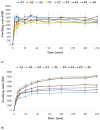
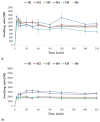
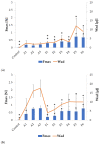
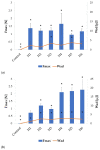
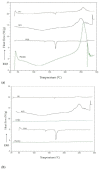
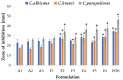
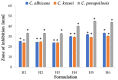
Fungal infections are a group of diseases which are challenging to treat because of drug-resistant fungi species, drug toxicity, and often severe patient conditions. Hence, research into new treatments, including new therapeutic substances and novel drug delivery systems, is being performed. Mucoadhesive dosage forms are beneficial to improving drug bioavailability by prolonging the residence time at the site of application. Sodium alginate is a natural polymer with favorable mucoadhesive and gelling properties, although its precipitation in acidic pH significantly disrupts the process of drug release in gastric conditions. Hypromellose is a hydrophilic, semi-synthetic cellulose derivative with mucoadhesive properties, which is widely used as a control release agent in pharmaceutical technology. The aim of this study was to evaluate the impact of hypromellose on alginate microparticles with posaconazole, designed to modify drug release and to improve their mucoadhesive properties for both oral or vaginal application.
Keywords: alginate; hypromellose; microparticles; mucoadhesive formulations; posaconazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Hard Gelatin Capsules with Alginate-Hypromellose Microparticles as a Multicompartment Drug Delivery System for Sustained Posaconazole Release.Int J Mol Sci. 2024 Jun 28;25(13):7116. doi: 10.3390/ijms25137116. Int J Mol Sci. 2024. PMID: 39000223 Free PMC article.
-
Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole.Mar Drugs. 2019 Dec 9;17(12):692. doi: 10.3390/md17120692. Mar Drugs. 2019. PMID: 31835313 Free PMC article.
-
Mucoadhesive tablets for the vaginal delivery of progesterone: in vitro evaluation and pharmacokinetics/pharmacodynamics in female rabbits.Drug Dev Ind Pharm. 2018 Feb;44(2):224-232. doi: 10.1080/03639045.2017.1386203. Epub 2017 Oct 23. Drug Dev Ind Pharm. 2018. PMID: 28956650
-
Hypromellose - A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery.J Control Release. 2020 Aug 10;324:695-727. doi: 10.1016/j.jconrel.2020.05.045. Epub 2020 May 29. J Control Release. 2020. PMID: 32479845 Review.
-
Alginate microparticles as oral colon drug delivery device: A review.Carbohydr Polym. 2017 Jul 15;168:32-43. doi: 10.1016/j.carbpol.2017.03.033. Epub 2017 Mar 14. Carbohydr Polym. 2017. PMID: 28457455 Review.
Cited by
-
Hard Gelatin Capsules with Alginate-Hypromellose Microparticles as a Multicompartment Drug Delivery System for Sustained Posaconazole Release.Int J Mol Sci. 2024 Jun 28;25(13):7116. doi: 10.3390/ijms25137116. Int J Mol Sci. 2024. PMID: 39000223 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

