Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I
- PMID: 37445982
- PMCID: PMC10341885
- DOI: 10.3390/ijms241310797
Accelerated Bone Loss in Transgenic Mice Expressing Constitutively Active TGF-β Receptor Type I
Abstract
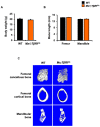
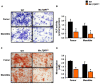
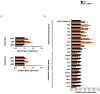
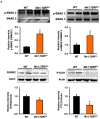
Transforming growth factor beta (TGF-β) is a key factor mediating the intercellular crosstalk between the hematopoietic stem cells and their microenvironment. Here, we investigated the skeletal phenotype of transgenic mice expressing constitutively active TGF-β receptor type I under the control of Mx1-Cre (Mx1;TβRICA mice). μCT analysis showed decreased cortical thickness, and cancellous bone volume in both femurs and mandibles. Histomorphometric analysis confirmed a decrease in cancellous bone volume due to increased osteoclast number and decreased osteoblast number. Primary osteoblasts showed decreased ALP and mineralization. Constitutive TβRI activation increased osteoclast differentiation. qPCR analysis showed that Tnfsf11/Tnfrsf11b ratio, Ctsk, Sufu, and Csf1 were increased whereas Runx2, Ptch1, and Ptch2 were decreased in Mx1;TβRICA femurs. Interestingly, Gli1, Wnt3a, Sp7, Alpl, Ptch1, Ptch2, and Shh mRNA expression were reduced whereas Tnfsf11/Tnfrsf11b ratio was increased in Mx1;TβRICA mandibles. Similarly, osteoclast-related genes were increased in Mx1;TβRICA osteoclasts whereas osteoblast-related genes were reduced in Mx1;TβRICA osteoblasts. Western blot analysis indicated that SMAD2 and SMAD3 phosphorylation was increased in Mx1;TβRICA osteoblasts, and SMAD3 phosphorylation was increased in Mx1;TβRICA osteoclasts. CTSK was increased while RUNX2 and PTCH1 was decreased in Mx1;TβRICA mice. Microindentation analysis indicated decreased hardness in Mx1;TβRICA mice. Our study indicated that Mx1;TβRICA mice were osteopenic by increasing osteoclast number and decreasing osteoblast number, possibly by suppressing Hedgehog signaling pathways.
Keywords: TGF-β; bone; hardness; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Differential Gene Expression Involved in Bone Turnover of Mice Expressing Constitutively Active TGFβ Receptor Type I.Int J Mol Sci. 2024 May 27;25(11):5829. doi: 10.3390/ijms25115829. Int J Mol Sci. 2024. PMID: 38892016 Free PMC article.
-
Nephrectomy Induces Severe Bone Loss in Mice Expressing Constitutively Active TGFβ Receptor Type I.Int J Mol Sci. 2025 Mar 17;26(6):2704. doi: 10.3390/ijms26062704. Int J Mol Sci. 2025. PMID: 40141345 Free PMC article.
-
Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone.PLoS One. 2009;4(4):e5275. doi: 10.1371/journal.pone.0005275. Epub 2008 Apr 16. PLoS One. 2009. PMID: 19357790 Free PMC article.
-
Repetitive exposure to TGF-beta suppresses TGF-beta type I receptor expression by differentiated osteoblasts.Gene. 2006 Sep 1;379:175-84. doi: 10.1016/j.gene.2006.05.005. Epub 2006 May 19. Gene. 2006. PMID: 16806744
-
Regulatory mechanisms of osteoblast and osteoclast differentiation.Oral Dis. 2002 May;8(3):147-59. doi: 10.1034/j.1601-0825.2002.01829.x. Oral Dis. 2002. PMID: 12108759 Review.
Cited by
-
Advances in Bone Biology.Int J Mol Sci. 2024 Jun 23;25(13):6892. doi: 10.3390/ijms25136892. Int J Mol Sci. 2024. PMID: 39000002 Free PMC article.
-
Differential Gene Expression Involved in Bone Turnover of Mice Expressing Constitutively Active TGFβ Receptor Type I.Int J Mol Sci. 2024 May 27;25(11):5829. doi: 10.3390/ijms25115829. Int J Mol Sci. 2024. PMID: 38892016 Free PMC article.
-
Nephrectomy Induces Severe Bone Loss in Mice Expressing Constitutively Active TGFβ Receptor Type I.Int J Mol Sci. 2025 Mar 17;26(6):2704. doi: 10.3390/ijms26062704. Int J Mol Sci. 2025. PMID: 40141345 Free PMC article.
References
-
- Edwards J.R., Nyman J.S., Lwin S.T., Moore M.M., Esparza J., O’Quinn E.C., Hart A.J., Biswas S., Patil C.A., Lonning S., et al. Inhibition of TGF-β signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J. Bone Min. Res. 2010;25:2419–2426. doi: 10.1002/jbmr.139. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

