Research Progress of Benzothiazole and Benzoxazole Derivatives in the Discovery of Agricultural Chemicals
- PMID: 37445983
- PMCID: PMC10341659
- DOI: 10.3390/ijms241310807
Research Progress of Benzothiazole and Benzoxazole Derivatives in the Discovery of Agricultural Chemicals
Abstract
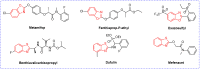
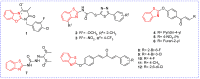
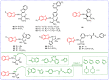
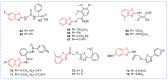
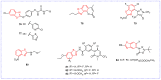
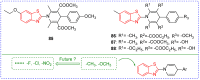
Benzoxazole and benzothiazole have a broad spectrum of agricultural biological activities, such as antibacterial, antiviral, and herbicidal activities, which are important fused heterocyclic scaffold structures in agrochemical discovery. In recent years, great progress has been made in the research of benzoxazoles and benzothiazoles, especially in the development of herbicides and insecticides. With the widespread use of benzoxazoles and benzothiazoles, there may be more new products containing benzoxazoles and benzothiazoles in the future. We systematically reviewed the application of benzoxazoles and benzothiazoles in discovering new agrochemicals in the past two decades and summarized the antibacterial, fungicidal, antiviral, herbicidal, and insecticidal activities of the active compounds. We also discussed the structural-activity relationship and mechanism of the active compounds. This work aims to provide inspiration and ideas for the discovery of new agrochemicals based on benzoxazole and benzothiazole.
Keywords: SAR; agrochemical; benzothiazole; benzoxazole; mechanism.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
-
- Evidente A., Cimmino A., Masi M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019;18:843–870. doi: 10.1007/s11101-019-09624-0. - DOI
-
- Chen Y.F., Luo X., Wang Y., Xing Z.F., Peng J., Chen J.X. Design, synthesis and antibacterial activity of 1,3,4-oxadiazole sufones containing sulfonamide structure. Chin. J. Org. Chem. 2023;43:274–284. doi: 10.6023/cjoc202204068. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

