Comparison of Monoamine Oxidase-A, Aβ Plaques, Tau, and Translocator Protein Levels in Postmortem Human Alzheimer's Disease Brain
- PMID: 37445985
- PMCID: PMC10341404
- DOI: 10.3390/ijms241310808
Comparison of Monoamine Oxidase-A, Aβ Plaques, Tau, and Translocator Protein Levels in Postmortem Human Alzheimer's Disease Brain
Abstract
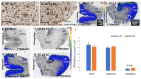
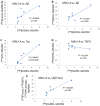
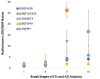
Increased monoamine oxidase-A (MAO-A) activity in Alzheimer's disease (AD) may be detrimental to the point of neurodegeneration. To assess MAO-A activity in AD, we compared four biomarkers, Aβ plaques, tau, translocator protein (TSPO), and MAO-A in postmortem AD. Radiotracers were [18F]FAZIN3 for MAO-A, [18F]flotaza and [125I]IBETA for Aβ plaques, [124/125I]IPPI for tau, and [18F]FEPPA for TSPO imaging. Brain sections of the anterior cingulate (AC; gray matter GM) and corpus callosum (CC; white matter WM) from cognitively normal control (CN, n = 6) and AD (n = 6) subjects were imaged using autoradiography and immunostaining. Using competition with clorgyline and (R)-deprenyl, the binding of [18F]FAZIN3 was confirmed to be selective to MAO-A levels in the AD brain sections. Increases in MAO-A, Aβ plaque, tau, and TSPO activity were found in the AD brains compared to the control brains. The [18F]FAZIN3 ratio in AD GM versus CN GM was 2.80, suggesting a 180% increase in MAO-A activity. Using GM-to-WM ratios of AD versus CN, a >50% increase in MAO-A activity was observed (AD/CN = 1.58). Linear positive correlations of [18F]FAZIN3 with [18F]flotaza, [125I]IBETA, and [125I]IPPI were measured and suggested an increase in MAO-A activity with increases in Aβ plaques and tau activity. Our results support the finding that MAO-A activity is elevated in the anterior cingulate cortex in AD and thus may provide a new biomarker for AD in this brain region.
Keywords: Alzheimer’s disease; Aβ plaques; [125I]IPPI; [18F]FEPPA; [18F]flotaza; [18]FAZIN3; human tau; monoamine oxidase-A.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Patterson C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers. Alzheimer’s Disease International (ADI); London, UK: 2018.
-
- Mendez P.C., Surace E., Bérgamo Y., Calandri I., Vázquez S., Sevlever G., Allegri R.F. Biomarkers for Alzheimers disease. Where we stand and where we are headed. Medicina. 2019;79:546–551. - PubMed
-
- Thijssen E.H., La Joie R., Wolf A., Strom A., Wang P., Iaccarino L., Bourakova V., Cobigo Y., Heuer H., Spina S., et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020;26:387–397. doi: 10.1038/s41591-020-0762-2. - DOI - PMC - PubMed

