The Oral Transglutaminase 2 Inhibitor ZED1227 Accumulates in the Villous Enterocytes in Celiac Disease Patients during Gluten Challenge and Drug Treatment
- PMID: 37445994
- PMCID: PMC10341493
- DOI: 10.3390/ijms241310815
The Oral Transglutaminase 2 Inhibitor ZED1227 Accumulates in the Villous Enterocytes in Celiac Disease Patients during Gluten Challenge and Drug Treatment
Abstract
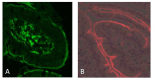
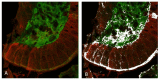
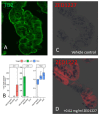
The enzyme transglutaminase 2 (TG2) plays a key role in celiac disease (CeD) pathogenesis. Active TG2 is located mainly extracellularly in the lamina propria but also in the villous enterocytes of the duodenum. The TG2 inhibitor ZED1227 is a promising drug candidate for treating CeD and is designed to block the TG2-catalyzed deamidation and crosslinking of gliadin peptides. Our aim was to study the accumulation of ZED1227 after oral administration of the drug. We studied duodenal biopsies derived from a phase 2a clinical drug trial using an antibody that detects ZED1227 when bound to the catalytic center of TG2. Human epithelial organoids were studied in vitro for the effect of ZED1227 on the activity of TG2 using the 5-biotin-pentylamine assay. The ZED1227-TG2 complex was found mainly in the villous enterocytes in post-treatment biopsies. The signal of ZED1227-TG2 was strongest in the luminal epithelial brush border, while the intensity of the signal in the lamina propria was only ~20% of that in the villous enterocytes. No signal specific to ZED1227 could be detected in pretreatment biopsies or in biopsies from patients randomized to the placebo treatment arm. ZED1227-TG2 staining co-localized with total TG2 and native and deamidated gliadin peptides on the enterocyte luminal surface. Inhibition of TG2 activity by ZED1227 was demonstrated in epithelial organoids. Our findings suggest that active TG2 is present at the luminal side of the villous epithelium and that inhibition of TG2 activity by ZED1227 occurs already there before gliadin peptides enter the lamina propria.
Keywords: brush border; celiac disease therapy; enterocyte; gliadin; gluten.
Conflict of interest statement
J.I. is the owner of Jilab Inc. M.H. and R.P. are employees and shareholders of Zedira and co-inventors of a patent covering the ZED1227 compound. T.Z., R.M. and R.G. are employees of DrFalkPharma Gmbh. M.M. is the owner and Chair of Board at Maki HealthTech Ltd. (MHT). MHT receives management/Advisory Affiliation fees from Dr. Falk Pharma, Topas Therapeutics, Calypso Biotech, Vaccitech, ImmunogenX, and Equillium; holds patents licensed to Labsystems Diagnostics, from which MHT receives royalties via Tampere University Hospital.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

