Building a Scaffold for Arteriovenous Fistula Maturation: Unravelling the Role of the Extracellular Matrix
- PMID: 37446003
- PMCID: PMC10341877
- DOI: 10.3390/ijms241310825
Building a Scaffold for Arteriovenous Fistula Maturation: Unravelling the Role of the Extracellular Matrix
Abstract
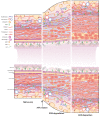
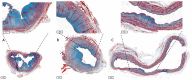
Vascular access is the lifeline for patients receiving haemodialysis as kidney replacement therapy. As a surgically created arteriovenous fistula (AVF) provides a high-flow conduit suitable for cannulation, it remains the vascular access of choice. In order to use an AVF successfully, the luminal diameter and the vessel wall of the venous outflow tract have to increase. This process is referred to as AVF maturation. AVF non-maturation is an important limitation of AVFs that contributes to their poor primary patency rates. To date, there is no clear overview of the overall role of the extracellular matrix (ECM) in AVF maturation. The ECM is essential for vascular functioning, as it provides structural and mechanical strength and communicates with vascular cells to regulate their differentiation and proliferation. Thus, the ECM is involved in multiple processes that regulate AVF maturation, and it is essential to study its anatomy and vascular response to AVF surgery to define therapeutic targets to improve AVF maturation. In this review, we discuss the composition of both the arterial and venous ECM and its incorporation in the three vessel layers: the tunica intima, media, and adventitia. Furthermore, we examine the effect of chronic kidney failure on the vasculature, the timing of ECM remodelling post-AVF surgery, and current ECM interventions to improve AVF maturation. Lastly, the suitability of ECM interventions as a therapeutic target for AVF maturation will be discussed.
Keywords: AVF maturation; arteriovenous fistula; extracellular matrix; vascular remodelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schmidli J., Widmer M.K., Basile C., de Donato G., Gallieni M., Gibbons C.P., Haage P., Hamilton G., Hedin U., Kamper L., et al. Editor’s Choice—Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2018;55:757–818. doi: 10.1016/j.ejvs.2018.02.001. - DOI - PubMed
-
- Astor B.C., Eustace J.A., Powe N.R., Klag M.J., Fink N.E., Coresh J., Josef Coresh for the CHOICE Study Type of Vascular Access and Survival among Incident Hemodialysis Patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J. Am. Soc. Nephrol. 2005;16:1449–1455. doi: 10.1681/ASN.2004090748. - DOI - PubMed
-
- Banerjee T., Kim S.J., Astor B., Shafi T., Coresh J., Powe N.R. Vascular Access Type, Inflammatory Markers, and Mortality in Incident Hemodialysis Patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am. J. Kidney Dis. 2014;64:954–961. doi: 10.1053/j.ajkd.2014.07.010. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

