Bortezomib Increased Vascular Permeability by Decreasing Cell-Cell Junction Molecules in Human Pulmonary Microvascular Endothelial Cells
- PMID: 37446020
- PMCID: PMC10342080
- DOI: 10.3390/ijms241310842
Bortezomib Increased Vascular Permeability by Decreasing Cell-Cell Junction Molecules in Human Pulmonary Microvascular Endothelial Cells
Abstract
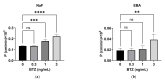
Bortezomib (BTZ), a chemotherapeutic drug used to treat multiple myeloma, induces life-threatening side effects, including severe pulmonary toxicity. However, the mechanisms underlying these effects remain unclear. The objectives of this study were to (1) investigate whether BTZ influences vascular permeability and (2) clarify the effect of BTZ on the expression of molecules associated with cell-cell junctions using human pulmonary microvascular endothelial cells in vitro. Clinically relevant concentrations of BTZ induced limited cytotoxicity and increased the permeability of human pulmonary microvascular endothelial cell monolayers. BTZ decreased the protein expression of claudin-5, occludin, and VE-cadherin but not that of ZO-1 and β-catenin. Additionally, BTZ decreased the mRNA expression of claudin-5, occludin, ZO-1, VE-cadherin, and β-catenin. Our results suggest that BTZ increases the vascular permeability of the pulmonary microvascular endothelium by downregulating cell-cell junction molecules, particularly claudin-5, occludin, and VE-cadherin.
Keywords: adhesion molecule; bortezomib; vascular permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hideshima T., Richardson P., Chauhan D., Palombella V.J., Elliott P.J., Adams J., Anderson K.C. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

