The Slow Progression of Diabetic Retinopathy Is Associated with Transient Protection of Retinal Vessels from Death
- PMID: 37446043
- PMCID: PMC10341443
- DOI: 10.3390/ijms241310869
The Slow Progression of Diabetic Retinopathy Is Associated with Transient Protection of Retinal Vessels from Death
Abstract
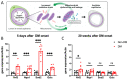
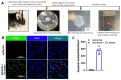
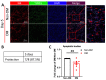
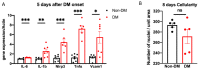
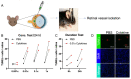
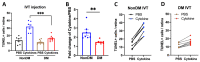
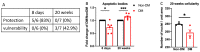
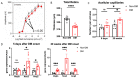
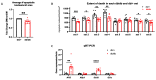
The purpose of this study was to investigate the reason that diabetic retinopathy (DR) is delayed from the onset of diabetes (DM) in diabetic mice. To this end, we tested the hypothesis that the deleterious effects of DM are initially tolerated because endogenous antioxidative defense is elevated and thereby confers resistance to oxidative stress-induced death. We found that this was indeed the case in both type 1 DM (T1D) and type 2 DM (T2D) mouse models. The retinal expression of antioxidant defense genes was increased soon after the onset of DM. In addition, ischemia/oxidative stress caused less death in the retinal vasculature of DM versus non-DM mice. Further investigation with T1D mice revealed that protection was transient; it waned as the duration of DM was prolonged. Finally, a loss of protection was associated with the manifestation of both neural and vascular abnormalities that are diagnostic of DR in mice. These observations demonstrate that DM can transiently activate protection from oxidative stress, which is a plausible explanation for the delay in the development of DR from the onset of DM.
Keywords: diabetic retinopathy; oxidative stress; protection from diabetic retinopathy; retinal capillaries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy.Int J Mol Sci. 2025 May 29;26(11):5211. doi: 10.3390/ijms26115211. Int J Mol Sci. 2025. PMID: 40508021 Free PMC article. Review.
-
Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats.Mol Biol Rep. 2012 Apr;39(4):3727-35. doi: 10.1007/s11033-011-1148-9. Epub 2011 Sep 28. Mol Biol Rep. 2012. PMID: 21952821
-
Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy.Cochrane Database Syst Rev. 2023 Feb 22;2(2):CD013775. doi: 10.1002/14651858.CD013775.pub2. Cochrane Database Syst Rev. 2023. PMID: 36815723 Free PMC article. Review.
-
Iron Overload Accelerates the Progression of Diabetic Retinopathy in Association with Increased Retinal Renin Expression.Sci Rep. 2018 Feb 14;8(1):3025. doi: 10.1038/s41598-018-21276-2. Sci Rep. 2018. PMID: 29445185 Free PMC article.
-
Reduced light exposure mitigates streptozotocin-induced vascular changes and gliosis in diabetic retina by an anti-inflammatory effect and increased retinal cholesterol turnover.Chem Biol Interact. 2024 May 1;394:110996. doi: 10.1016/j.cbi.2024.110996. Epub 2024 Apr 7. Chem Biol Interact. 2024. PMID: 38593908
Cited by
-
An Assay to Detect Protection of the Retinal Vasculature from Diabetes-Related Death in Mice.J Vis Exp. 2024 Jan 12;(203):10.3791/66123. doi: 10.3791/66123. J Vis Exp. 2024. PMID: 38284520 Free PMC article.
-
Diabetic Retinopathy-A Review.Curr Diabetes Rev. 2025;21(7):43-55. doi: 10.2174/0115733998296228240521151050. Curr Diabetes Rev. 2025. PMID: 38831577 Review.
-
Retinal Microvascular Alterations in a Patient with Type 1 Diabetes Mellitus, Hemoglobin D Hemoglobinopathy, and High Myopia-Case Report and Review of the Literature.Diagnostics (Basel). 2023 Sep 13;13(18):2934. doi: 10.3390/diagnostics13182934. Diagnostics (Basel). 2023. PMID: 37761301 Free PMC article.
-
Manifestation of Pathology in Animal Models of Diabetic Retinopathy Is Delayed from the Onset of Diabetes.Int J Mol Sci. 2024 Jan 28;25(3):1610. doi: 10.3390/ijms25031610. Int J Mol Sci. 2024. PMID: 38338889 Free PMC article. Review.
-
The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy.Int J Mol Sci. 2025 May 29;26(11):5211. doi: 10.3390/ijms26115211. Int J Mol Sci. 2025. PMID: 40508021 Free PMC article. Review.
References
-
- Teo Z.L., Tham Y.-C., Yu M., Chee M.L., Rim T.H., Cheung N., Bikbov M.M., Wang Y.X., Tang Y., Lu Y., et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580–1591. doi: 10.1016/j.ophtha.2021.04.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

