Regulatory Cues in Pulmonary Fibrosis-With Emphasis on the AIM2 Inflammasome
- PMID: 37446052
- PMCID: PMC10341703
- DOI: 10.3390/ijms241310876
Regulatory Cues in Pulmonary Fibrosis-With Emphasis on the AIM2 Inflammasome
Abstract
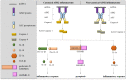
Pulmonary fibrosis (PF) is a chronic lung disorder characterized by the presence of scarred and thickened lung tissues. Although the Food and Drug Administration approved two antifibrotic drugs, pirfenidone, and nintedanib, that are currently utilized for treating idiopathic PF (IPF), the clinical therapeutic efficacy remains unsatisfactory. It is crucial to develop new drugs or treatment schemes that combine pirfenidone or nintedanib to achieve more effective outcomes for PF patients. Understanding the complex mechanisms underlying PF could potentially facilitate drug discovery. Previous studies have found that the activation of inflammasomes, including nucleotide-binding and oligomerization domain (NOD)-like receptor protein (NLRP)1, NLRP3, NOD-like receptor C4, and absent in melanoma (AIM)2, contributes to lung inflammation and fibrosis. This article aims to summarize the cellular and molecular regulatory cues that contribute to PF with a particular emphasis on the role of AIM2 inflammasome in mediating pathophysiologic events during PF development. The insights gained from this research may pave the way for the development of more effective strategies for the prevention and treatment of PF.
Keywords: AIM2 inflammasome; epithelial-mesenchymal transition; inflammation; pulmonary fibrosis; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

