GSK-3 Inhibitor Elraglusib Enhances Tumor-Infiltrating Immune Cell Activation in Tumor Biopsies and Synergizes with Anti-PD-L1 in a Murine Model of Colorectal Cancer
- PMID: 37446056
- PMCID: PMC10342141
- DOI: 10.3390/ijms241310870
GSK-3 Inhibitor Elraglusib Enhances Tumor-Infiltrating Immune Cell Activation in Tumor Biopsies and Synergizes with Anti-PD-L1 in a Murine Model of Colorectal Cancer
Abstract
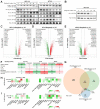
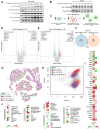
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase that has been implicated in numerous oncogenic processes. GSK-3 inhibitor elraglusib (9-ING-41) has shown promising preclinical and clinical antitumor activity across multiple tumor types. Despite promising early-phase clinical trial results, there have been limited efforts to characterize the potential immunomodulatory properties of elraglusib. We report that elraglusib promotes immune cell-mediated tumor cell killing of microsatellite stable colorectal cancer (CRC) cells. Mechanistically, elraglusib sensitized CRC cells to immune-mediated cytotoxicity and enhanced immune cell effector function. Using western blots, we found that elraglusib decreased CRC cell expression of NF-κB p65 and several survival proteins. Using microarrays, we discovered that elraglusib upregulated the expression of proapoptotic and antiproliferative genes and downregulated the expression of cell proliferation, cell cycle progression, metastasis, TGFβ signaling, and anti-apoptotic genes in CRC cells. Elraglusib reduced CRC cell production of immunosuppressive molecules such as VEGF, GDF-15, and sPD-L1. Elraglusib increased immune cell IFN-γ secretion, which upregulated CRC cell gasdermin B expression to potentially enhance pyroptosis. Elraglusib enhanced immune effector function resulting in augmented granzyme B, IFN-γ, TNF-α, and TRAIL production. Using a syngeneic, immunocompetent murine model of microsatellite stable CRC, we evaluated elraglusib as a single agent or combined with immune checkpoint blockade (anti-PD-1/L1) and observed improved survival in the elraglusib and anti-PD-L1 group. Murine responders had increased tumor-infiltrating T cells, augmented granzyme B expression, and fewer regulatory T cells. Murine responders had reduced immunosuppressive (VEGF, VEGFR2) and elevated immunostimulatory (GM-CSF, IL-12p70) cytokine plasma concentrations. To determine the clinical significance, we then utilized elraglusib-treated patient plasma samples and found that reduced VEGF and BAFF and elevated IL-1 beta, CCL22, and CCL4 concentrations correlated with improved survival. Using paired tumor biopsies, we found that tumor-infiltrating immune cells had a reduced expression of inhibitory immune checkpoints (VISTA, PD-1, PD-L2) and an elevated expression of T-cell activation markers (CTLA-4, OX40L) after elraglusib treatment. These results address a significant gap in knowledge concerning the immunomodulatory mechanisms of GSK-3 inhibitor elraglusib, provide a rationale for the clinical evaluation of elraglusib in combination with immune checkpoint blockade, and are expected to have an impact on additional tumor types, besides CRC.
Keywords: 9-ING-41; GSK-3; elraglusib; immune checkpoint blockade; immunotherapy.
Conflict of interest statement
Elraglusib (9-ING-41) has been licensed to Actuate Therapeutics. K.E.H., P.S., and W.S.E-D. receive research funding for preclinical studies from Actuate Therapeutics, Inc (March 2022- Feb. 2024). B.A.C. received institutional funding for a clinical trial related to 9-ING-41 from Actuate Therapeutics, Inc. F.J.G. has served as a consultant to Actuate Therapeutics, Inc. Daniel Newhouse is an employee and shareholder of NanoString Technologies Inc. All remaining authors report no disclosures.
Figures





Update of
-
GSK-3 inhibitor elraglusib enhances tumor-infiltrating immune cell activation in tumor biopsies and synergizes with anti-PD-L1 in a murine model of colorectal cancer.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527499. doi: 10.1101/2023.02.07.527499. bioRxiv. 2023. Update in: Int J Mol Sci. 2023 Jun 29;24(13):10870. doi: 10.3390/ijms241310870. PMID: 36798357 Free PMC article. Updated. Preprint.
Similar articles
-
GSK-3 inhibitor elraglusib enhances tumor-infiltrating immune cell activation in tumor biopsies and synergizes with anti-PD-L1 in a murine model of colorectal cancer.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527499. doi: 10.1101/2023.02.07.527499. bioRxiv. 2023. Update in: Int J Mol Sci. 2023 Jun 29;24(13):10870. doi: 10.3390/ijms241310870. PMID: 36798357 Free PMC article. Updated. Preprint.
-
Elraglusib (9-ING-41), a selective small-molecule inhibitor of glycogen synthase kinase-3 beta, reduces expression of immune checkpoint molecules PD-1, TIGIT and LAG-3 and enhances CD8+ T cell cytolytic killing of melanoma cells.J Hematol Oncol. 2022 Sep 14;15(1):134. doi: 10.1186/s13045-022-01352-x. J Hematol Oncol. 2022. PMID: 36104795 Free PMC article.
-
Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer.J Immunother Cancer. 2021 Jan;9(1):e001895. doi: 10.1136/jitc-2020-001895. J Immunother Cancer. 2021. PMID: 33462141 Free PMC article.
-
Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities.Front Immunol. 2020 Nov 5;11:598877. doi: 10.3389/fimmu.2020.598877. eCollection 2020. Front Immunol. 2020. PMID: 33250900 Free PMC article. Review.
-
PD-1/PD-L1 inhibitors for early and middle stage microsatellite high-instability and stable colorectal cancer: a review.Int J Colorectal Dis. 2024 May 29;39(1):83. doi: 10.1007/s00384-024-04654-3. Int J Colorectal Dis. 2024. PMID: 38809459 Free PMC article. Review.
Cited by
-
Elraglusib Induces Cytotoxicity via Direct Microtubule Destabilization Independently of GSK3 Inhibition.Cancer Res Commun. 2024 Nov 1;4(11):3013-3024. doi: 10.1158/2767-9764.CRC-24-0408. Cancer Res Commun. 2024. PMID: 39470360 Free PMC article.
-
Comment on: Glycogen synthase kinase 3 controls T-cell exhaustion by regulating NFAT activation.Cell Mol Immunol. 2025 May;22(5):557-558. doi: 10.1038/s41423-025-01277-8. Epub 2025 Apr 2. Cell Mol Immunol. 2025. PMID: 40175728 No abstract available.
-
Phase II study of elraglusib (9-ING-41), a GSK-3β inhibitor, in combination with gemcitabine plus nab-paclitaxel in previously untreated metastatic pancreatic cancer.ESMO Open. 2025 Jun;10(6):105122. doi: 10.1016/j.esmoop.2025.105122. Epub 2025 May 21. ESMO Open. 2025. PMID: 40403387 Free PMC article. Clinical Trial.
-
Imipridones ONC201/ONC206 + RT/TMZ triple (IRT) therapy reduces intracranial tumor burden, prolongs survival in orthotopic IDH-WT GBM mouse model, and suppresses MGMT.Oncotarget. 2025 Mar 27;16:230-248. doi: 10.18632/oncotarget.28707. Oncotarget. 2025. PMID: 40145650 Free PMC article.
-
Tumor microenvironment-modulating oncolytic adenovirus combined with GSK-3β inhibitor enhances antitumor immune response against bladder cancer.Front Immunol. 2024 May 15;15:1360436. doi: 10.3389/fimmu.2024.1360436. eCollection 2024. Front Immunol. 2024. PMID: 38812516 Free PMC article.
References
-
- Ding L., Madamsetty V.S., Kiers S., Alekhina O., Ugolkov A., Dube J., Zhang Y., Zhang J.S., Wang E., Dutta S.K., et al. Glycogen Synthase Kinase-3 Inhibition Sensitizes Pancreatic Cancer Cells to Chemotherapy by Abrogating the TopBP1/ATR-Mediated DNA Damage Response. Clin. Cancer Res. 2019;25:6452–6462. doi: 10.1158/1078-0432.CCR-19-0799. - DOI - PMC - PubMed
-
- Zhang J.S., Herreros-Villanueva M., Koenig A., Deng Z., de Narvajas A.A.M., Gomez T.S., Meng X., Bujanda L., Ellenrieder V., Li X.K., et al. Differential activity of GSK-3 isoforms regulates NF-κB and TRAIL- or TNFα induced apoptosis in pancreatic cancer cells. Cell Death Dis. 2014;5:e1142. doi: 10.1038/cddis.2014.102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

