Microfluidics and Organoids, the Power Couple of Developmental Biology and Oncology Studies
- PMID: 37446057
- PMCID: PMC10341942
- DOI: 10.3390/ijms241310882
Microfluidics and Organoids, the Power Couple of Developmental Biology and Oncology Studies
Abstract
Organoids are an advanced cell model that hold the key to unlocking a deeper understanding of in vivo cellular processes. This model can be used in understanding organ development, disease progression, and treatment efficacy. As the scientific world embraces the model, it must also establish the best practices for cultivating organoids and utilizing them to the greatest potential in assays. Microfluidic devices are emerging as a solution to overcome the challenges of organoids and adapt assays. Unfortunately, the various applications of organoids often depend on specific features in a device. In this review, we discuss the options and considerations for features and materials depending on the application and development of the organoid.
Keywords: microfluidic device; organoid filtering; organoid formation; organoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
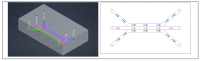

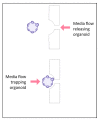


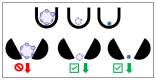
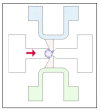



Similar articles
-
Engineering Vascularized Organoid-on-a-Chip Models.Annu Rev Biomed Eng. 2021 Jul 13;23:141-167. doi: 10.1146/annurev-bioeng-090120-094330. Epub 2021 Mar 23. Annu Rev Biomed Eng. 2021. PMID: 33756087
-
Developmentally inspired human 'organs on chips'.Development. 2018 May 18;145(16):dev156125. doi: 10.1242/dev.156125. Development. 2018. PMID: 29776965 Free PMC article.
-
Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation.Adv Biol (Weinh). 2021 Jun;5(6):e2000024. doi: 10.1002/adbi.202000024. Epub 2021 Apr 15. Adv Biol (Weinh). 2021. PMID: 33856745 Free PMC article. Review.
-
Microfluidic Platform for Generating and Releasing Patient-Derived Cancer Organoids with Diverse Shapes: Insight into Shape-Dependent Tumor Growth.Adv Mater. 2024 Nov;36(44):e2410547. doi: 10.1002/adma.202410547. Epub 2024 Sep 14. Adv Mater. 2024. PMID: 39276011
-
Engineering mechanobiology through organoids-on-chip: A strategy to boost therapeutics.J Tissue Eng Regen Med. 2021 Nov;15(11):883-899. doi: 10.1002/term.3234. Epub 2021 Aug 12. J Tissue Eng Regen Med. 2021. PMID: 34339588 Review.
Cited by
-
Advancing organ-on-chip systems: the role of microfluidics in neuro-cardiac research.Curr Res Pharmacol Drug Discov. 2025 Jul 3;9:100227. doi: 10.1016/j.crphar.2025.100227. eCollection 2025. Curr Res Pharmacol Drug Discov. 2025. PMID: 40689159 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

