Untangling Macropore Formation and Current Facilitation in P2X7
- PMID: 37446075
- PMCID: PMC10341454
- DOI: 10.3390/ijms241310896
Untangling Macropore Formation and Current Facilitation in P2X7
Abstract
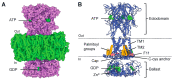
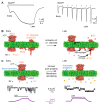
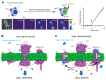
Macropore formation and current facilitation are intriguing phenomena associated with ATP-gated P2X7 receptors (P2X7). Macropores are large pores formed in the cell membrane that allow the passage of large molecules. The precise mechanisms underlying macropore formation remain poorly understood, but recent evidence suggests two alternative pathways: a direct entry through the P2X7 pore itself, and an indirect pathway triggered by P2X7 activation involving additional proteins, such as TMEM16F channel/scramblase. On the other hand, current facilitation refers to the progressive increase in current amplitude and activation kinetics observed with prolonged or repetitive exposure to ATP. Various mechanisms, including the activation of chloride channels and intrinsic properties of P2X7, have been proposed to explain this phenomenon. In this comprehensive review, we present an in-depth overview of P2X7 current facilitation and macropore formation, highlighting new findings and proposing mechanistic models that may offer fresh insights into these untangled processes.
Keywords: ATP sensitization; P2X7; current facilitation; macropore formation.
Conflict of interest statement
T.G. is a consultant with Bellus Health Cough Inc. The authors declare no conflict of interest.
Figures
Similar articles
-
P2X7 Receptors and TMEM16 Channels Are Functionally Coupled with Implications for Macropore Formation and Current Facilitation.Int J Mol Sci. 2021 Jun 18;22(12):6542. doi: 10.3390/ijms22126542. Int J Mol Sci. 2021. PMID: 34207150 Free PMC article.
-
Contribution of the Juxtatransmembrane Intracellular Regions to the Time Course and Permeation of ATP-gated P2X7 Receptor Ion Channels.J Biol Chem. 2015 Jun 5;290(23):14556-66. doi: 10.1074/jbc.M115.642033. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903136 Free PMC article.
-
The Elusive P2X7 Macropore.Trends Cell Biol. 2018 May;28(5):392-404. doi: 10.1016/j.tcb.2018.01.005. Epub 2018 Feb 10. Trends Cell Biol. 2018. PMID: 29439897 Review.
-
Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation.Br J Pharmacol. 2006 Feb;147(3):324-34. doi: 10.1038/sj.bjp.0706559. Br J Pharmacol. 2006. PMID: 16341234 Free PMC article.
-
Human P2X7 receptors - Properties of single ATP-gated ion channels.Biochem Pharmacol. 2021 May;187:114307. doi: 10.1016/j.bcp.2020.114307. Epub 2020 Oct 29. Biochem Pharmacol. 2021. PMID: 33130127 Review.
Cited by
-
Ionotropic purinergic receptor 7 (P2X7) channel structure and pharmacology provides insight regarding non-nucleotide agonism.Channels (Austin). 2024 Dec;18(1):2355150. doi: 10.1080/19336950.2024.2355150. Epub 2024 May 19. Channels (Austin). 2024. PMID: 38762911 Free PMC article. Review.
-
Purine and purinergic receptors in health and disease.MedComm (2020). 2023 Sep 7;4(5):e359. doi: 10.1002/mco2.359. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37692109 Free PMC article. Review.
-
The Purinergic P2X7 Receptor as a Target for Adjunctive Treatment for Drug-Refractory Epilepsy.Int J Mol Sci. 2024 Jun 23;25(13):6894. doi: 10.3390/ijms25136894. Int J Mol Sci. 2024. PMID: 39000004 Free PMC article. Review.
-
Microglia-induced neuroinflammation in hippocampal neurogenesis following traumatic brain injury.Heliyon. 2024 Aug 8;10(16):e35869. doi: 10.1016/j.heliyon.2024.e35869. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220913 Free PMC article. Review.
-
New insights into pathogenisis and therapies of P2X7R in Parkinson's disease.NPJ Parkinsons Dis. 2025 May 5;11(1):108. doi: 10.1038/s41531-025-00980-7. NPJ Parkinsons Dis. 2025. PMID: 40325043 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

