Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[ d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists
- PMID: 37446093
- PMCID: PMC10341893
- DOI: 10.3390/ijms241310918
Motifs in Natural Products as Useful Scaffolds to Obtain Novel Benzo[ d]imidazole-Based Cannabinoid Type 2 (CB2) Receptor Agonists
Abstract
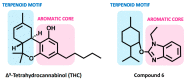
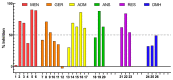
The endocannabinoid system (ECS) constitutes a broad-spectrum modulator of homeostasis in mammals, providing therapeutic opportunities for several pathologies. Its two main receptors, cannabinoid type 1 (CB1) and type 2 (CB2) receptors, mediate anti-inflammatory responses; however, their differing patterns of expression make the development of CB2-selective ligands therapeutically more attractive. The benzo[d]imidazole ring is considered to be a privileged scaffold in drug discovery and has demonstrated its versatility in the development of molecules with varied pharmacologic properties. On the other hand, the main psychoactive component of Cannabis sativa, delta-9-tetrahydrocannabinol (THC), can be structurally described as an aliphatic terpenoid motif fused to an aromatic polyphenolic (resorcinol) structure. Inspired by the structure of this phytocannabinoid, we combined different natural product motifs with a benzo[d]imidazole scaffold to obtain a new library of compounds targeting the CB2 receptor. Here, we synthesized 26 new compounds, out of which 15 presented CB2 binding and 3 showed potent agonist activity. SAR analysis indicated that the presence of bulky aliphatic or aromatic natural product motifs at position 2 of the benzo[d]imidazoles ring linked by an electronegative atom is essential for receptor recognition, while substituents with moderate bulkiness at position 1 of the heterocyclic core also participate in receptor recognition. Compounds 5, 6, and 16 were further characterized through in vitro cAMP functional assay, showing potent EC50 values between 20 and 3 nM, and compound 6 presented a significant difference between the EC50 of pharmacologic activity (3.36 nM) and IC50 of toxicity (30-38 µM).
Keywords: CB2 agonists; benzimidazoles; cannabinoid receptor; natural products; synthesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


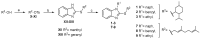


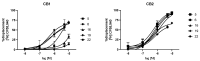


Similar articles
-
Structure-Based Identification of Potent Natural Product Chemotypes as Cannabinoid Receptor 1 Inverse Agonists.Molecules. 2018 Oct 13;23(10):2630. doi: 10.3390/molecules23102630. Molecules. 2018. PMID: 30322136 Free PMC article.
-
Design, synthesis, and structure-activity relationships of diindolylmethane derivatives as cannabinoid CB2 receptor agonists.Arch Pharm (Weinheim). 2023 Mar;356(3):e2200493. doi: 10.1002/ardp.202200493. Epub 2022 Nov 27. Arch Pharm (Weinheim). 2023. PMID: 36437108
-
Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes.Biochem Pharmacol. 2018 Nov;157:148-158. doi: 10.1016/j.bcp.2018.08.046. Epub 2018 Sep 6. Biochem Pharmacol. 2018. PMID: 30194918
-
Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products.Int J Mol Sci. 2020 Jul 17;21(14):5064. doi: 10.3390/ijms21145064. Int J Mol Sci. 2020. PMID: 32709050 Free PMC article. Review.
-
Synthetic cannabinoid receptor agonists: classification and nomenclature.Clin Toxicol (Phila). 2020 Feb;58(2):82-98. doi: 10.1080/15563650.2019.1661425. Epub 2019 Sep 16. Clin Toxicol (Phila). 2020. PMID: 31524007 Review.
Cited by
-
Investigation of photophysical properties and potential biological applications of substituted tris(polypyridyl)ruthenium(II) complexes.Front Chem. 2025 Feb 3;13:1491598. doi: 10.3389/fchem.2025.1491598. eCollection 2025. Front Chem. 2025. PMID: 39963354 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

