Cryopreservation Induces Acetylation of Metabolism-Related Proteins in Boar Sperm
- PMID: 37446160
- PMCID: PMC10341673
- DOI: 10.3390/ijms241310983
Cryopreservation Induces Acetylation of Metabolism-Related Proteins in Boar Sperm
Abstract
Cryodamage affects the normal physiological functions and survivability of boar sperm during cryopreservation. Lysine acetylation is thought to be an important regulatory mechanism in sperm functions. However, little is known about protein acetylation and its effects on cryotolerance or cryodamage in boar sperm. In this study, the characterization and protein acetylation dynamics of boar sperm during cryopreservation were determined using liquid chromatography-mass spectrometry (LC-MS). A total of 1440 proteins were identified out of 4705 modified proteins, and 2764 quantifiable sites were elucidated. Among the differentially modified sites, 1252 were found to be upregulated compared to 172 downregulated sites in fresh and frozen sperms. Gene ontology indicated that these differentially modified proteins are involved in metabolic processes and catalytic and antioxidant activities, which are involved in pyruvate metabolism, phosphorylation and lysine degradation. In addition, the present study demonstrated that the mRNA and protein expressions of SIRT5, IDH2, MDH2 and LDHC, associated with sperm quality parameters, are downregulated after cryopreservation. In conclusion, cryopreservation induces the acetylation and deacetylation of energy metabolism-related proteins, which may contribute to the post-thawed boar sperm quality parameters.
Keywords: boar; energy metabolism; protein acetylation; sperm cryopreservation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
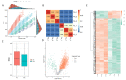


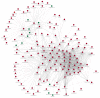
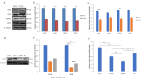
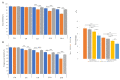


References
-
- Flores E., Ramió-Lluch L., Bucci D., Fernández-Novell J.M., Peña A., Rodríguez-Gil J.E. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology. 2011;76:1450–1464. doi: 10.1016/j.theriogenology.2011.05.039. - DOI - PubMed
-
- Evans H.C., Briggs E.F., Burnett R.H., Contreras-Correa Z.E., Duvic M.A., Dysart L.M., Gilmore A.A., Messman R.D., Reid D., Rasit Ugur M., et al. Harnessing the value of reproductive hormones in cattle production with considerations to animal welfare and human health. J. Anim. Sci. 2022;100:skac177. doi: 10.1093/jas/skac177. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

