Humanization of the Reaction Specificity of Mouse Alox15b Inversely Modified the Susceptibility of Corresponding Knock-In Mice in Two Different Animal Inflammation Models
- PMID: 37446212
- PMCID: PMC10341735
- DOI: 10.3390/ijms241311034
Humanization of the Reaction Specificity of Mouse Alox15b Inversely Modified the Susceptibility of Corresponding Knock-In Mice in Two Different Animal Inflammation Models
Abstract
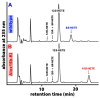
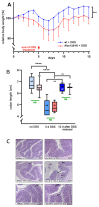
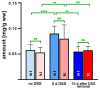
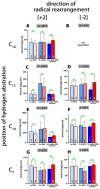
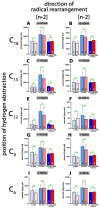
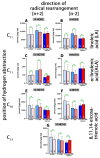
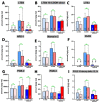
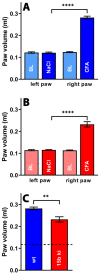
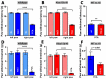
Mammalian arachidonic acid lipoxygenases (ALOXs) have been implicated in the pathogenesis of inflammatory diseases, and its pro- and anti-inflammatory effects have been reported for different ALOX-isoforms. Human ALOX15B oxygenates arachidonic acid to its 15-hydroperoxy derivative, whereas the corresponding 8-hydroperoxide is formed by mouse Alox15b (Alox8). This functional difference impacts the biosynthetic capacity of the two enzymes for creating pro- and anti-inflammatory eicosanoids. To explore the functional consequences of the humanization of the reaction specificity of mouse Alox15b in vivo, we tested Alox15b knock-in mice that express the arachidonic acid 15-lipoxygenating Tyr603Asp and His604Val double mutant of Alox15b, instead of the arachidonic acid 8-lipoxygenating wildtype enzyme, in two different animal inflammation models. In the dextran sodium sulfate-induced colitis model, female Alox15b-KI mice lost significantly more bodyweight during the acute phase of inflammation and recovered less rapidly during the resolution phase. Although we observed significant differences in the colonic levels of selected pro- and anti-inflammatory eicosanoids during the time-course of inflammation, there were no differences between the two genotypes at any time-point of the disease. In Freund's complete adjuvant-induced paw edema model, Alox15b-KI mice were less susceptible than outbred wildtype controls, though we did not observe significant differences in pain perception (Hargreaves-test, von Frey-test) when the two genotypes were compared. our data indicate that humanization of the reaction specificity of mouse Alox15b (Alox8) sensitizes mice for dextran sodium sulfate-induced experimental colitis, but partly protects the animals in the complete Freund's adjuvant-induced paw edema model.
Keywords: atherosclerosis; eicosanoids; fatty acids; inflammation; lipids; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Transgenic mice overexpressing human ALOX15 under the control of the aP2 promoter are partly protected in the complete Freund's adjuvant-induced paw inflammation model.Inflamm Res. 2023 Aug;72(8):1649-1664. doi: 10.1007/s00011-023-01770-8. Epub 2023 Jul 27. Inflamm Res. 2023. PMID: 37498393 Free PMC article.
-
Functional humanization of 15-lipoxygenase-1 (Alox15) protects mice from dextran sodium sulfate induced intestinal inflammation.Cell Mol Biol Lett. 2025 Jul 13;30(1):81. doi: 10.1186/s11658-025-00756-0. Cell Mol Biol Lett. 2025. PMID: 40653455 Free PMC article.
-
Murine Alox8 versus the human ALOX15B ortholog: differences and similarities.Pflugers Arch. 2024 Dec;476(12):1817-1832. doi: 10.1007/s00424-024-02961-w. Epub 2024 Apr 19. Pflugers Arch. 2024. PMID: 38637408 Free PMC article. Review.
-
Male Knock-in Mice Expressing an Arachidonic Acid Lipoxygenase 15B (Alox15B) with Humanized Reaction Specificity Are Prematurely Growth Arrested When Aging.Biomedicines. 2022 Jun 10;10(6):1379. doi: 10.3390/biomedicines10061379. Biomedicines. 2022. PMID: 35740398 Free PMC article.
-
Regulation and Functions of 15-Lipoxygenases in Human Macrophages.Front Pharmacol. 2019 Jul 4;10:719. doi: 10.3389/fphar.2019.00719. eCollection 2019. Front Pharmacol. 2019. PMID: 31333453 Free PMC article. Review.
Cited by
-
Transgenic mice overexpressing human ALOX15 under the control of the aP2 promoter are partly protected in the complete Freund's adjuvant-induced paw inflammation model.Inflamm Res. 2023 Aug;72(8):1649-1664. doi: 10.1007/s00011-023-01770-8. Epub 2023 Jul 27. Inflamm Res. 2023. PMID: 37498393 Free PMC article.
-
Functional humanization of 15-lipoxygenase-1 (Alox15) protects mice from dextran sodium sulfate induced intestinal inflammation.Cell Mol Biol Lett. 2025 Jul 13;30(1):81. doi: 10.1186/s11658-025-00756-0. Cell Mol Biol Lett. 2025. PMID: 40653455 Free PMC article.
-
Murine Alox8 versus the human ALOX15B ortholog: differences and similarities.Pflugers Arch. 2024 Dec;476(12):1817-1832. doi: 10.1007/s00424-024-02961-w. Epub 2024 Apr 19. Pflugers Arch. 2024. PMID: 38637408 Free PMC article. Review.
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
-
Multiple Roles of Apolipoprotein E4 in Oxidative Lipid Metabolism and Ferroptosis During the Pathogenesis of Alzheimer's Disease.J Mol Neurosci. 2024 Jul 3;74(3):62. doi: 10.1007/s12031-024-02224-4. J Mol Neurosci. 2024. PMID: 38958788 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

