Cryopreservation of Ovarian and Testicular Tissue and the Influence on Epigenetic Pattern
- PMID: 37446239
- PMCID: PMC10341714
- DOI: 10.3390/ijms241311061
Cryopreservation of Ovarian and Testicular Tissue and the Influence on Epigenetic Pattern
Abstract
Ovarian tissue cryopreservation (OTC) or testicular tissue cryopreservation (TTC) are effective and often the only options for fertility preservation in female or male patients due to oncological, medical, or social aspects. While TTC and resumption of spermatogenesis, either in vivo or in vitro, has still be considered an experimental approach in humans, OTC and autotransplantation has been applied increasingly to preserve fertility, with more than 200 live births worldwide. However, the cryopreservation of reproductive cells followed by the resumption of gametogenesis, either in vivo or in vitro, may interfere with sensitive and highly regulated cellular processes. In particular, the epigenetic profile, which includes not just reversible modifications of the DNA itself but also post-translational histone modifications, small non-coding RNAs, gene expression and availability, and storage of related proteins or transcripts, have to be considered in this context. Due to complex reprogramming and maintenance mechanisms of the epigenome in germ cells, growing embryos, and offspring, OTC and TTC are carried out at very critical moments early in the life cycle. Given this background, the safety of OTC and TTC, taking into account the epigenetic profile, has to be clarified. Cryopreservation of mature germ cells (including metaphase II oocytes and mature spermatozoa collected via ejaculation or more invasively after testicular biopsy) or embryos has been used successfully for many years in medically assisted reproduction (MAR). However, tissue freezing followed by in vitro or in vivo gametogenesis has become more attractive in the past, while few human studies have analysed the epigenetic effects, with most data deriving from animal studies. In this review, we highlight the potential influence of the cryopreservation of immature germ cells and subsequent in vivo or in vitro growth and differentiation on the epigenetic profile (including DNA methylation, post-translational histone modifications, and the abundance and availability of relevant transcripts and proteins) in humans and animals.
Keywords: epigenetic; genomic imprinting; medically assisted reproduction; ovarian tissue cryopreservation; testicular tissue cryopreservation.
Conflict of interest statement
The authors declare no conflict of interest. The authors declare that this study received funding from Theramex Germany GmbH. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Figures

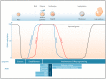
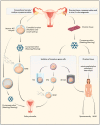

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

