Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a
- PMID: 37446248
- PMCID: PMC10342269
- DOI: 10.3390/ijms241311070
Dye Decolorization by a Miniaturized Peroxidase Fe-MimochromeVI*a
Abstract
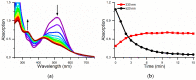
Oxidases and peroxidases have found application in the field of chlorine-free organic dye degradation in the paper, toothpaste, and detergent industries. Nevertheless, their widespread use is somehow hindered because of their cost, availability, and batch-to-batch reproducibility. Here, we report the catalytic proficiency of a miniaturized synthetic peroxidase, Fe-Mimochrome VI*a, in the decolorization of four organic dyes, as representatives of either the heterocyclic or triarylmethane class of dyes. Fe-Mimochrome VI*a performed over 130 turnovers in less than five minutes in an aqueous buffer at a neutral pH under mild conditions.
Keywords: artificial metalloenzyme; bleaching; dye degradation; enzymatic treatment; peroxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






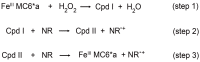
References
-
- Silva M.C., Torres J.A., Castro A.A., da Cunha E.F.F., Alves de Oliveira L.C., Corrêa A.D., Ramalho T.C. Combined Experimental and Theoretical Study on the Removal of Pollutant Compounds by Peroxidases: Affinity and Reactivity toward a Bioremediation Catalyst. J. Biomol. Struct. Dyn. 2016;34:1839–1848. doi: 10.1080/07391102.2015.1063456. - DOI - PubMed
-
- Regalado C., García-Almendárez B.E., Duarte-Vázquez M.A. Biotechnological Applications of Peroxidases. Phytochem. Rev. 2004;3:243–256. doi: 10.1023/B:PHYT.0000047797.81958.69. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

