A C57BL/6J Fancg-KO Mouse Model Generated by CRISPR/Cas9 Partially Captures the Human Phenotype
- PMID: 37446306
- PMCID: PMC10342586
- DOI: 10.3390/ijms241311129
A C57BL/6J Fancg-KO Mouse Model Generated by CRISPR/Cas9 Partially Captures the Human Phenotype
Abstract
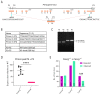
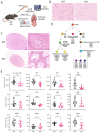
Fanconi anemia (FA) develops due to a mutation in one of the FANC genes that are involved in the repair of interstrand crosslinks (ICLs). FANCG, a member of the FA core complex, is essential for ICL repair. Previous FANCG-deficient mouse models were generated with drug-based selection cassettes in mixed mice backgrounds, leading to a disparity in the interpretation of genotype-related phenotype. We created a Fancg-KO (KO) mouse model using CRISPR/Cas9 to exclude these confounders. The entire Fancg locus was targeted and maintained on the immunological well-characterized C57BL/6J background. The intercrossing of heterozygous mice resulted in sub-Mendelian numbers of homozygous mice, suggesting the loss of FANCG can be embryonically lethal. KO mice displayed infertility and hypogonadism, but no other developmental problems. Bone marrow analysis revealed a defect in various hematopoietic stem and progenitor subsets with a bias towards myelopoiesis. Cell lines derived from Fancg-KO mice were hypersensitive to the crosslinking agents cisplatin and Mitomycin C, and Fancg-KO mouse embryonic fibroblasts (MEFs) displayed increased γ-H2AX upon cisplatin treatment. The reconstitution of these MEFs with Fancg cDNA corrected for the ICL hypersensitivity. This project provides a new, genetically, and immunologically well-defined Fancg-KO mouse model for further in vivo and in vitro studies on FANCG and ICL repair.
Keywords: CRISPR/Cas9; DNA damage response; FANCG; Mitomycin C (MMC); cisplatin (CsPt); fanconi anemia (FA); genetically engineered mouse model (GEMM); hematopoiesis; interstrand crosslink (ICL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice.Hum Mol Genet. 2002 Feb 1;11(3):273-81. doi: 10.1093/hmg/11.3.273. Hum Mol Genet. 2002. PMID: 11823446
-
Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9.Blood. 2001 Dec 1;98(12):3435-40. doi: 10.1182/blood.v98.12.3435. Blood. 2001. PMID: 11719385
-
Direct interaction of the Fanconi anaemia protein FANCG with BRCA2/FANCD1.Hum Mol Genet. 2003 Oct 1;12(19):2503-10. doi: 10.1093/hmg/ddg266. Epub 2003 Aug 5. Hum Mol Genet. 2003. PMID: 12915460
-
Current knowledge on the pathophysiology of Fanconi anemia: from genes to phenotypes.Int J Hematol. 2001 Jul;74(1):33-41. doi: 10.1007/BF02982547. Int J Hematol. 2001. PMID: 11530803 Review.
-
[Progress of research on protein composition and gene therapy of Fanconi anaemia - review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004 Apr;12(2):231-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004. PMID: 15157341 Review. Chinese.
Cited by
-
Dual role of proliferating cell nuclear antigen monoubiquitination in facilitating Fanconi anemia-mediated interstrand crosslink repair.PNAS Nexus. 2024 Jun 18;3(7):pgae242. doi: 10.1093/pnasnexus/pgae242. eCollection 2024 Jul. PNAS Nexus. 2024. PMID: 38957451 Free PMC article.
References
-
- Errazquin R., Page A., Suñol A., Segrelles C., Carrasco E., Peral J., Garrido-Aranda A., Del Marro S., Ortiz J., Lorz C., et al. Development of a mouse model for spontaneous oral squamous cell carcinoma in Fanconi anemia. Oral Oncol. 2022;134:106184. doi: 10.1016/j.oraloncology.2022.106184. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

