Effects of Voluntary Physical Exercise on the Neurovascular Unit in a Mouse Model of Alzheimer's Disease
- PMID: 37446312
- PMCID: PMC10342693
- DOI: 10.3390/ijms241311134
Effects of Voluntary Physical Exercise on the Neurovascular Unit in a Mouse Model of Alzheimer's Disease
Abstract
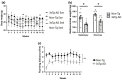
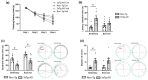
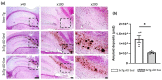
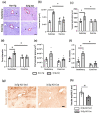
Alzheimer's disease (AD) is the most common neurodegenerative disorder worldwide. Histopathologically, AD presents two pathognomonic hallmarks: (1) neurofibrillary tangles, characterized by intracellular deposits of hyperphosphorylated tau protein, and (2) extracellular amyloid deposits (amyloid plaques) in the brain vasculature (cerebral amyloid angiopathy; CAA). It has been proposed that vascular amyloid deposits could trigger neurovascular unit (NVU) dysfunction in AD. The NVU is composed primarily of astrocytic feet, endothelial cells, pericytes, and basement membrane. Although physical exercise is hypothesized to have beneficial effects against AD, it is unknown whether its positive effects extend to ameliorating CAA and improving the physiology of the NVU. We used the triple transgenic animal model for AD (3xTg-AD) at 13 months old and analyzed through behavioral and histological assays, the effect of voluntary physical exercise on cognitive functions, amyloid angiopathy, and the NVU. Our results show that 3xTg-AD mice develop vascular amyloid deposits which correlate with cognitive deficits and NVU alteration. Interestingly, the physical exercise regimen decreases amyloid angiopathy and correlates with an improvement in cognitive function as well as in the underlying integrity of the NVU components. Physical exercise could represent a key therapeutic approach in cerebral amyloid angiopathy and NVU stability in AD patients.
Keywords: Alzheimer’s disease; astrocytic end-feet; basal membrane; cerebral amyloid angiopathy; cognition; neurovascular unit; pericytes; physical exercise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO. [(accessed on 10 June 2023)]. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dementia.
-
- Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

