The Impact of SRT2104 on Skeletal Muscle Mitochondrial Function, Redox Biology, and Loss of Muscle Mass in Hindlimb Unloaded Rats
- PMID: 37446313
- PMCID: PMC10342025
- DOI: 10.3390/ijms241311135
The Impact of SRT2104 on Skeletal Muscle Mitochondrial Function, Redox Biology, and Loss of Muscle Mass in Hindlimb Unloaded Rats
Abstract
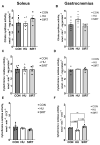
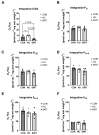
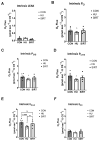
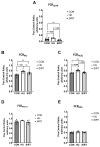
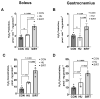
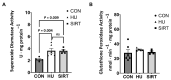
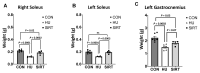
Mechanical unloading during microgravity causes skeletal muscle atrophy and impairs mitochondrial energetics. The elevated production of reactive oxygen species (ROS) by mitochondria and Nox2, coupled with impairment of stress protection (e.g., SIRT1, antioxidant enzymes), contribute to atrophy. We tested the hypothesis that the SIRT1 activator, SRT2104 would rescue unloading-induced mitochondrial dysfunction. Mitochondrial function in rat gastrocnemius and soleus muscles were evaluated under three conditions (10 days): ambulatory control (CON), hindlimb unloaded (HU), and hindlimb-unloaded-treated with SRT2104 (SIRT). Oxidative phosphorylation, electron transfer capacities, H2O2 production, and oxidative and antioxidant enzymes were quantified using high-resolution respirometry and colorimetry. In the gastrocnemius, (1) integrative (per mg tissue) proton LEAK was lesser in SIRT than in HU or CON; (2) intrinsic (relative to citrate synthase) maximal noncoupled electron transfer capacity (ECI+II) was lesser, while complex I-supported oxidative phosphorylation to ECI+II was greater in HU than CON; (3) the contribution of LEAK to ECI+II was greatest, but cytochrome c oxidase activity was lowest in HU. In both muscles, H2O2 production and concentration was greatest in SIRT, as was gastrocnemius superoxide dismutase activity. In the soleus, H2O2 concentration was greater in HU compared to CON. These results indicate that SRT2104 preserves mitochondrial function in unloaded skeletal muscle, suggesting its potential to support healthy muscle cells in microgravity by promoting necessary energy production in mitochondria.
Keywords: SRT2104; antioxidants; electron transfer system; hindlimb unloading; mitochondria; oxidative stress; respiration; skeletal muscle; spaceflight.
Conflict of interest statement
The authors declare no real or perceived conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

