Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity
- PMID: 37446444
- PMCID: PMC10343489
- DOI: 10.3390/nano13131928
Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity
Abstract
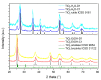
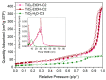
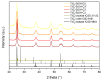
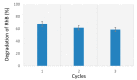
For this study, the synthesis of TiO2 nanomaterials was performed via a novel sol-gel method employing titanium butoxide as a metal precursor, Pluronic F127 as a templating agent, toluene as a swelling agent, and acidic water or ethanol as the reaction solvents. The method was designed by tailoring certain reaction parameters, such as the sequence of toluene addition, magnetic stirring, the type of reaction solvent, and the calcination conditions. Analysis of the specific surface area and porosity was carried out via N2 physisorption, whereas the morphological features of the solids were investigated via transmission electron microscopy. The crystalline structure of both the dried powders and the calcined materials was evaluated using X-ray diffraction analysis. It transpired that the different phase compositions of the solids are related to the specific synthesis medium employed. Under the adopted reaction conditions, ethanol, which was used as a reaction solvent, promoted the local arrangement of dispersed anatase particles, the specific arrangement of which does not lead to rutile transformation. Conversely, the use of water alone supported high-particle packing, evolving into a rutile phase. The photodegradation of Rhodamine B was used as a target reaction for testing the photocatalytic activity of the selected samples.
Keywords: anatase; photocatalysis; porous nanoparticles; rutile; sol-gel synthesis; titanium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Sun S., Song P., Cui J., Liang S. Amorphous TiO2 nanostructures: Synthesis, fundamental properties and photocatalytic applications. Catal. Sci. Technol. 2019;9:4198–4215. doi: 10.1039/C9CY01020C. - DOI
-
- Sippel C., Guaglianoni W.C., Bergmann C.P. Titanium Dioxide Nanomaterials for Renewable Energy Applications. In: Kopp Alves A., editor. Environmental Applications of Nanomaterials. Springer International Publishing; Cham, Switzerland: 2022. pp. 73–96. - DOI
-
- Kang X., Liu S., Dai Z., He Y., Song X., Tan Z. Titanium Dioxide: From Engineering to Applications. Catalysts. 2019;9:191. doi: 10.3390/catal9020191. - DOI
LinkOut - more resources
Full Text Sources

