Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity
- PMID: 37446500
- PMCID: PMC10343924
- DOI: 10.3390/nano13131984
Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity
Abstract
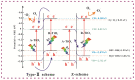
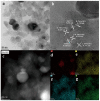
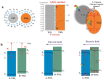
Plasmonic effect-enhanced Z-type heterojunction photocatalysts comprise a promising solution to the two fundamental problems of current TiO2-based photocatalysis concerning low-charge carrier separation efficiency and low utilization of solar illumination. A plasmonic effect-enhanced TiN@anatase-TiO2/rutile-TiO2 Z-type heterojunction photocatalyst with the strong interface of the N-O chemical bond was synthesized by hydrothermal oxidation of TiN. The prepared photocatalyst shows desirable visible light absorption and good visible-light-photocatalytic activity. The enhancement in photocatalytic activities contribute to the plasma resonance effect of TiN, the N-O bond-connected charge transfer channel at the TiO2/TiN heterointerface, and the synergistically Z-type charge transfer pathway between the anatase TiO2 (A-TiO2) and rutile TiO2 (R-TiO2). The optimization study shows that the catalyst with a weight ratio of A-TiO2/R-TiO2/TiN of approximately 15:1:1 achieved the best visible light photodegradation activity. This work demonstrates the effectiveness of fabricating plasmonic effect-enhanced Z-type heterostructure semiconductor photocatalysts with enhanced visible-light-photocatalytic activities.
Keywords: LSPR; TiO2; Z-type system; photocatalyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Masudy-Panah S., Siavash Moakhar R., Chua C.S., Kushwaha A., Dalapati G.K. Stable and Efficient CuO Based Photocathode through Oxygen-Rich Composition and Au–Pd Nanostructure Incorporation for Solar-Hydrogen Production. ACS Appl. Mater. Interfaces. 2017;9:27596–27606. doi: 10.1021/acsami.7b02685. - DOI - PubMed
-
- Takata T., Domen K. Particulate Photocatalysts for Water Splitting: Recent Advances and Future Prospects. ACS Energy Lett. 2019;4:542–549. doi: 10.1021/acsenergylett.8b02209. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

